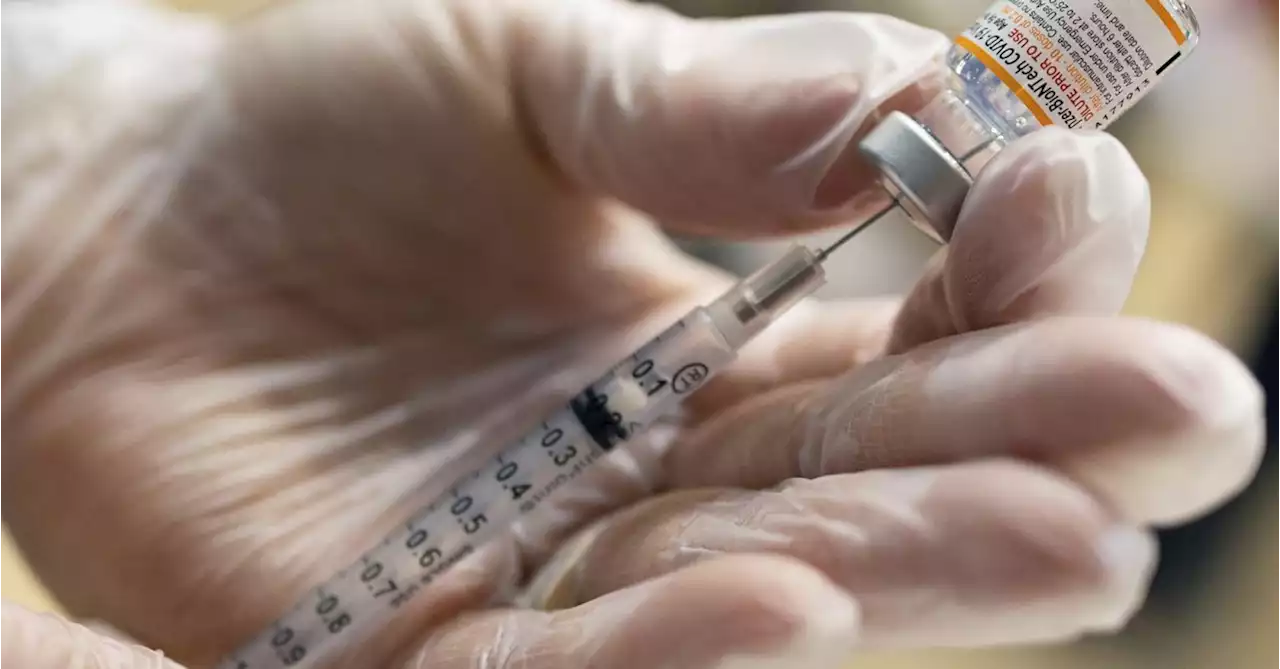
Pfizer Inc expects the latest results from a clinical trial for kids under the age of 5 of the COVID-19 vaccine it developed with Germany's BioNTech SE by April, a top company scientist said on Wednesday.
A vaccinator draws a Pfizer-BioNTech coronavirus disease pediatric vaccine in Lansdale, Pennsylvania, U.S., December 5, 2021. REUTERS/Hannah Beierexpects the latest results from a clinical trial for kids under the age of 5 of the COVID-19 vaccine it developed with Germany's BioNTech SE by April, a top company scientist said on Wednesday.
"The study has been amended to give a third dose to everybody who's less than five at least eight weeks after their last vaccination," Dr. Alejandra Gurtman, a Pfizer vaccine researcher said at a meeting of the U.S. Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices . She said the company aims to have data for the age group by the end of March or the beginning of April.
In December, Pfizer said it was changing the design of the trial because children between the ages of 2 and 4 who were given two 3-microgram doses of the vaccine did not have the same immune response that a larger dose of the vaccine generated in older children.Gurtman also said the company was studying a third dose of its vaccine in children ages 5 to 11, six months after their second dose.
The Pfizer-BioNTech vaccine is authorized in the United States for people age 5 and older. On Wednesday, ACIP backed booster shots of the vaccine for ages 12 to 15.Reporting by Michael Erman; Editing by Leslie Adler and Bill BerkrotSubscribe for our daily curated newsletter to receive the latest exclusive Reuters coverage delivered to your inbox.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 COVID-19 scams: How to prevent buying fake COVID testsIf you end up falling victim to a scam, you can report it to the FTC. Find out how.
COVID-19 scams: How to prevent buying fake COVID testsIf you end up falling victim to a scam, you can report it to the FTC. Find out how.
Consulte Mais informação »
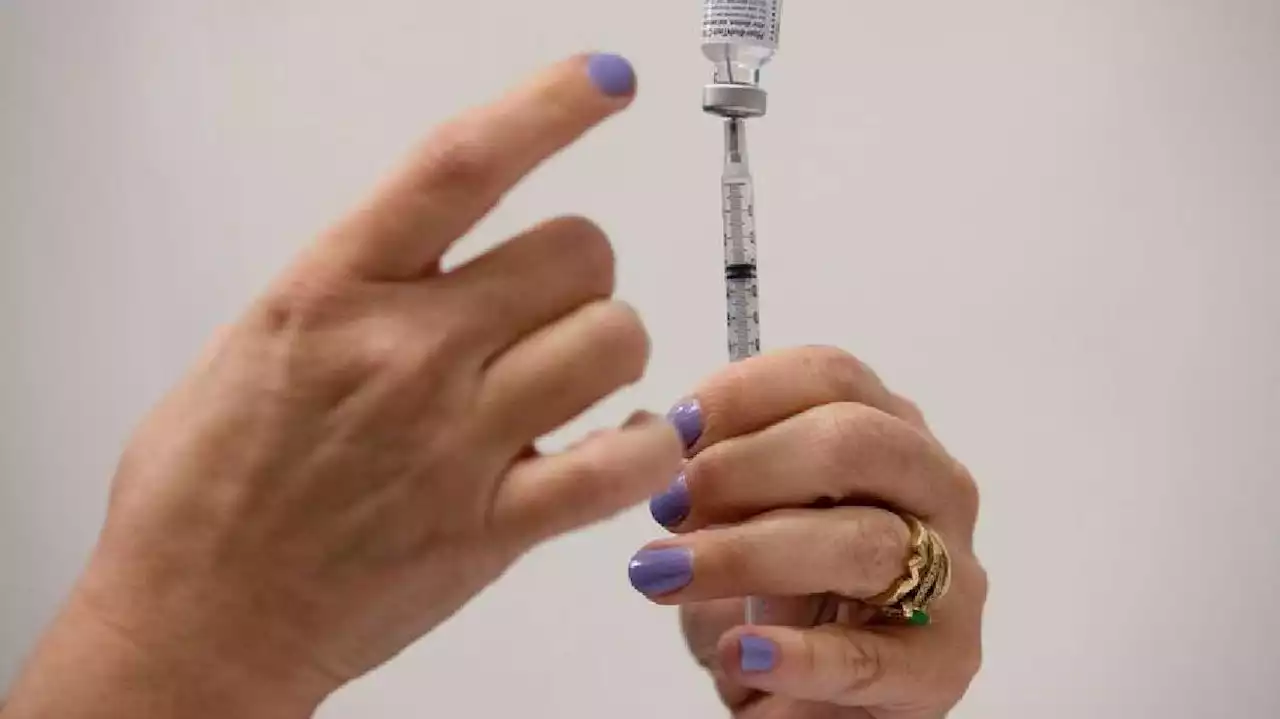 CDC recommends 5-month gap for Pfizer COVID-19 booster doseThe U.S. Centers for Disease Control and Prevention on Tuesday recommended shortening the interval between Pfizer-BioNTech's second COVID-19 vaccine dose and the booster shot to five months from six.
CDC recommends 5-month gap for Pfizer COVID-19 booster doseThe U.S. Centers for Disease Control and Prevention on Tuesday recommended shortening the interval between Pfizer-BioNTech's second COVID-19 vaccine dose and the booster shot to five months from six.
Consulte Mais informação »
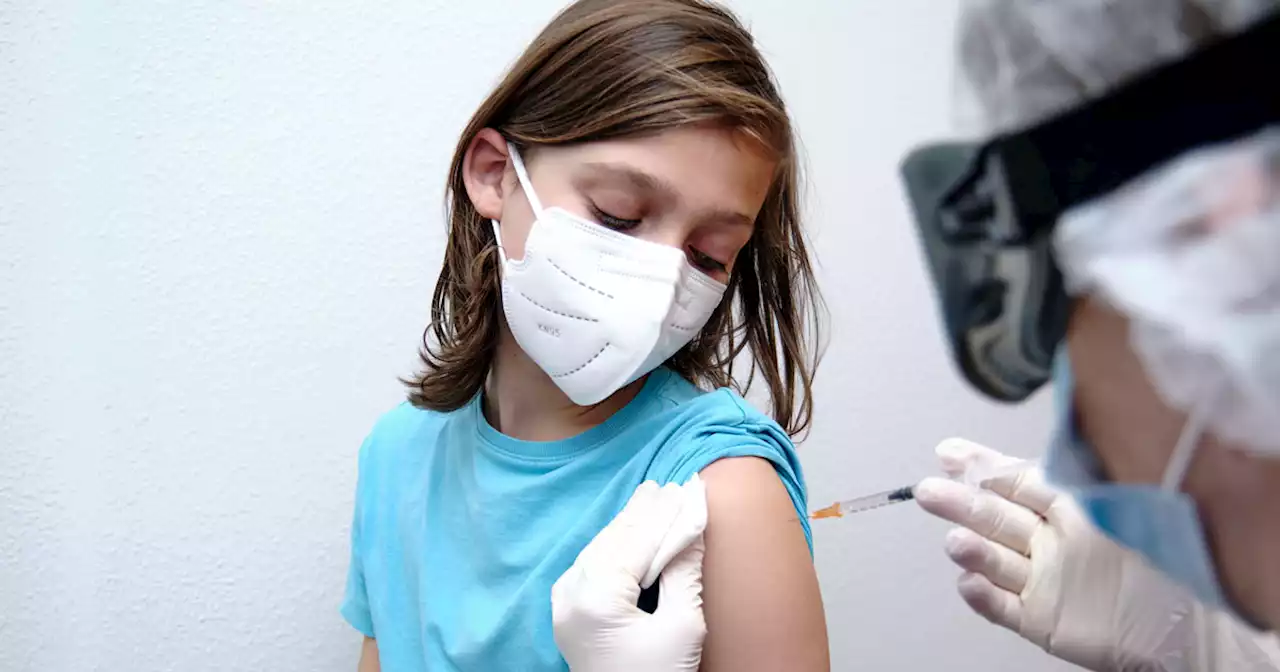 FDA Approves Pfizer COVID-19 Boosters For 12- to 15-Year-OldsThe FDA has approved COVID-19 booster for 12- to 15-year-olds as teens return to school amid the omicron surge.
FDA Approves Pfizer COVID-19 Boosters For 12- to 15-Year-OldsThe FDA has approved COVID-19 booster for 12- to 15-year-olds as teens return to school amid the omicron surge.
Consulte Mais informação »
 Pediatrician answers your questions on Pfizer Covid-19 booster for ages 12-15The FDA gave emergency use authorization for booster doses of Pfizer/BioNTech's Covid-19 vaccine for children ages 12 to 15 on Monday. A pediatrician answers parents' questions about the shots.
Pediatrician answers your questions on Pfizer Covid-19 booster for ages 12-15The FDA gave emergency use authorization for booster doses of Pfizer/BioNTech's Covid-19 vaccine for children ages 12 to 15 on Monday. A pediatrician answers parents' questions about the shots.
Consulte Mais informação »
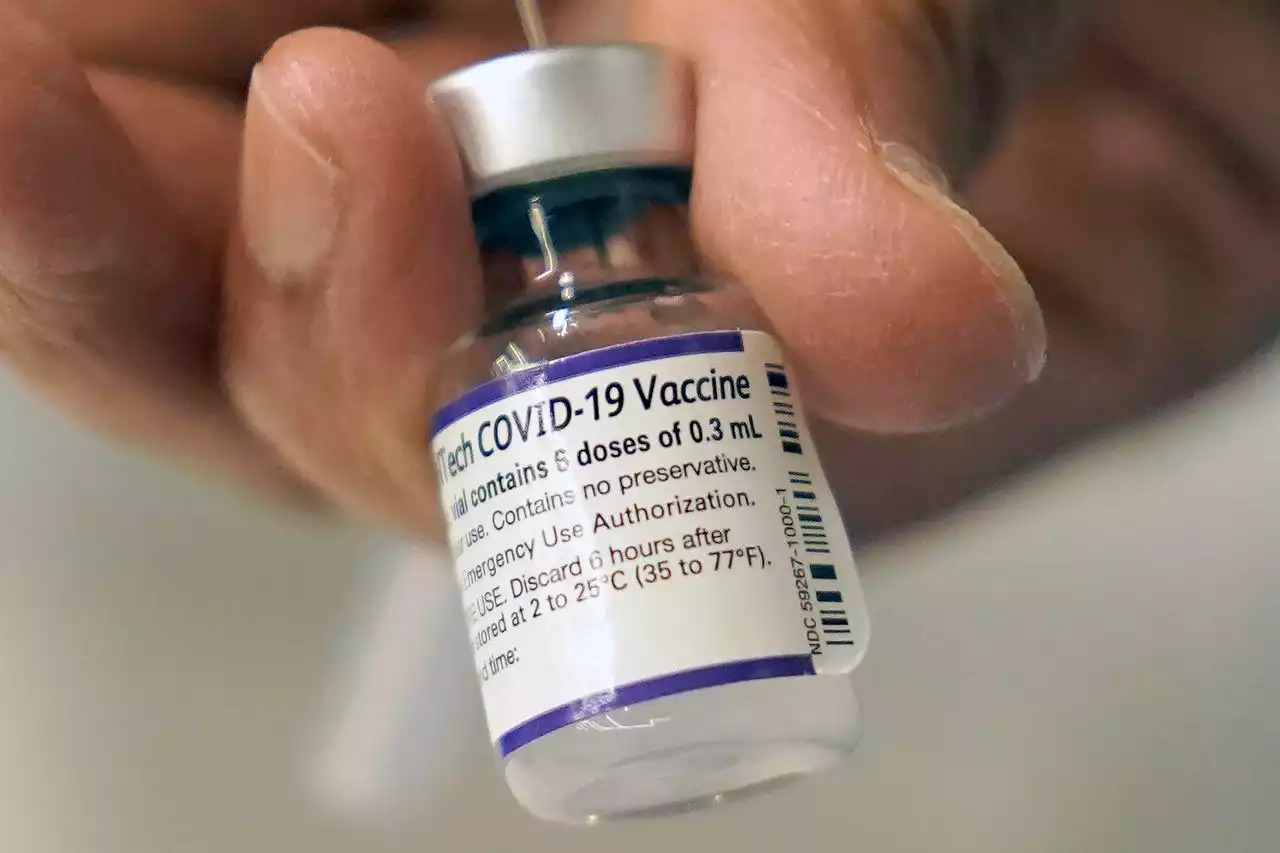 U.S. advisers support Pfizer COVID-19 boosters for younger teensThe CDC’s advisers were swayed by real-world U.S. data showing that symptomatic COVID-19 cases and hospitalizations are between seven and 11 times higher in unvaccinated adolescents than vaccinated ones.
U.S. advisers support Pfizer COVID-19 boosters for younger teensThe CDC’s advisers were swayed by real-world U.S. data showing that symptomatic COVID-19 cases and hospitalizations are between seven and 11 times higher in unvaccinated adolescents than vaccinated ones.
Consulte Mais informação »
 CDC panel endorses Pfizer COVID-19 vaccine booster for 12- to 15-year-oldsThe CDC’s director, Dr. Rochelle Walensky, will weigh the panel’s advice before making a final decision soon.
CDC panel endorses Pfizer COVID-19 vaccine booster for 12- to 15-year-oldsThe CDC’s director, Dr. Rochelle Walensky, will weigh the panel’s advice before making a final decision soon.
Consulte Mais informação »