The FDA gave emergency use authorization for booster doses of Pfizer/BioNTech's Covid-19 vaccine for children ages 12 to 15 on Monday. A pediatrician answers parents' questions about the shots.
The US Food and Drug Administration gave emergency use authorization for booster doses of Pfizer/BioNTech's Covid-19 vaccine for children ages 12 to 15 Monday.
FDA expands booster eligibility to adolescents and shortens time needed after primary seriesThe agency also shaved four weeks off the waiting period for the booster after the administration of the second dose, from at least six months to at least five months for everyone 12 and older."The FDA expanded emergency use to a whole group of people, children aged 12 to 15, who were not previously eligible for boosters," said pediatrician and child development expert Dr.
There is still this concern of cardiomyopathy, particularly in older teenagers. But even when we have seen children get this incredibly rare side effect, the vast majority have recovered with no long-term health issues. I think we can feel very good that, on the balance, the vaccine is a tremendous positive.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
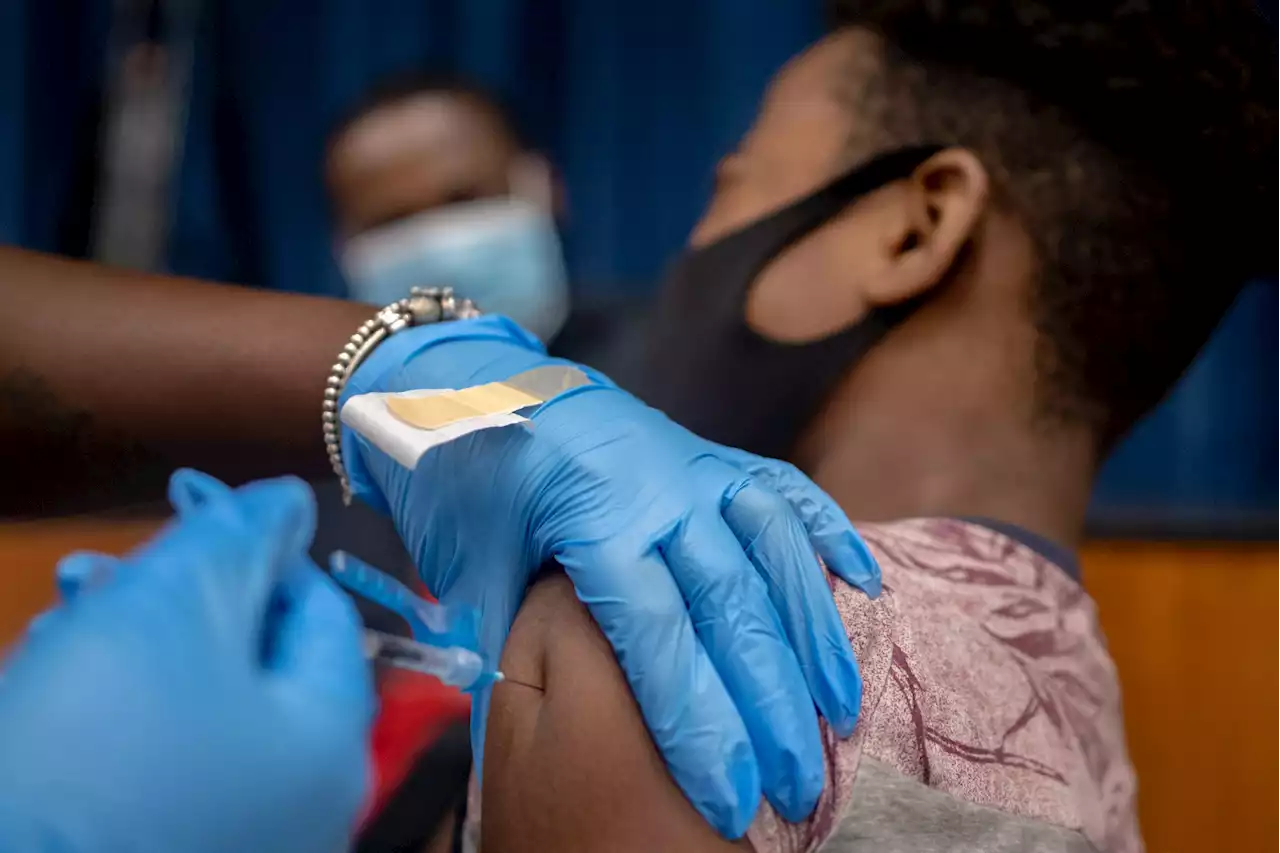 FDA authorizes Pfizer Covid-19 vaccine boosters for 12- to 15-year-oldsThe US kicked off 2022 amid a massive Covid-19 case spike — driven by the highly contagious Omicron variant — that some experts warn will be different than any other time in the pandemic.
FDA authorizes Pfizer Covid-19 vaccine boosters for 12- to 15-year-oldsThe US kicked off 2022 amid a massive Covid-19 case spike — driven by the highly contagious Omicron variant — that some experts warn will be different than any other time in the pandemic.
Consulte Mais informação »
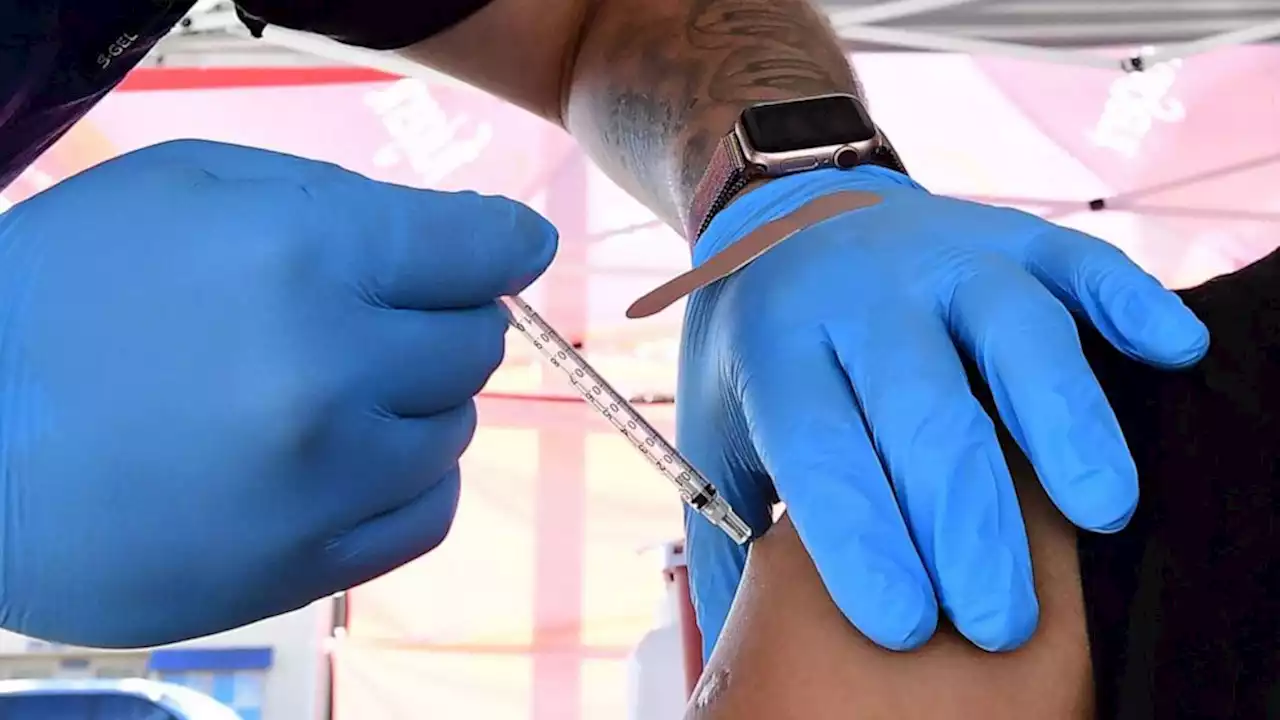 FDA authorizes Pfizer COVID-19 boosters for 12- to 15-year oldsThe FDA on Monday authorized Pfizer COVID-19 boosters for 12- to 15-year olds amid the omicron surge as children head back to school.
FDA authorizes Pfizer COVID-19 boosters for 12- to 15-year oldsThe FDA on Monday authorized Pfizer COVID-19 boosters for 12- to 15-year olds amid the omicron surge as children head back to school.
Consulte Mais informação »
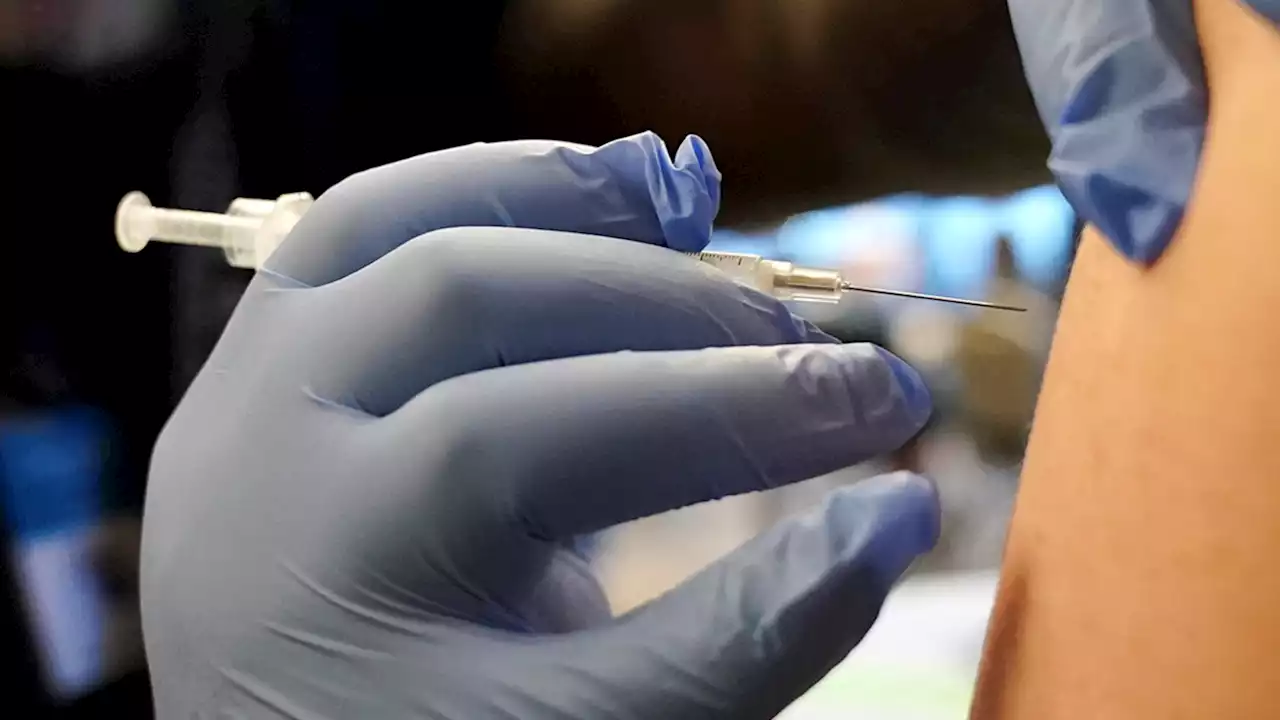 FDA expands Pfizer COVID-19 boosters for 12- to 15-year-olds as omicron surgesThe U.S. is expanding COVID-19 boosters as it confronts the omicron surge, with the FDA allowing extra Pfizer shots for children as young as 12.
FDA expands Pfizer COVID-19 boosters for 12- to 15-year-olds as omicron surgesThe U.S. is expanding COVID-19 boosters as it confronts the omicron surge, with the FDA allowing extra Pfizer shots for children as young as 12.
Consulte Mais informação »
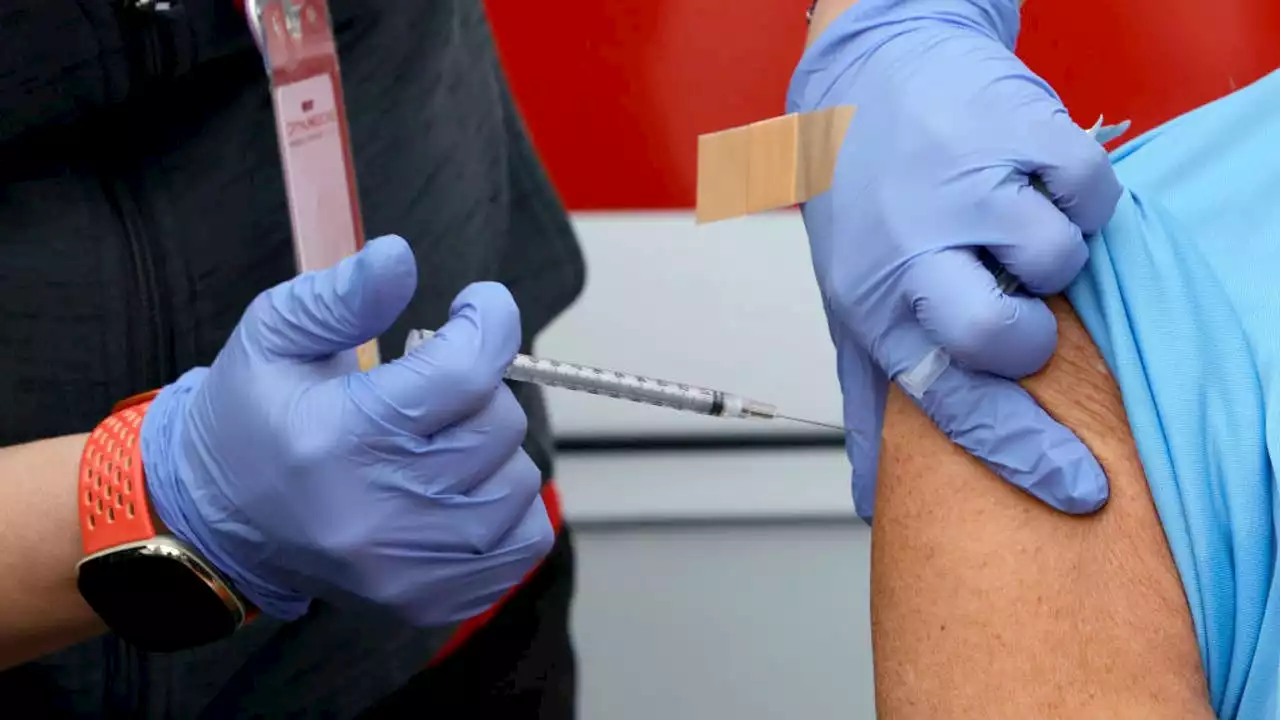 FDA expands Pfizer COVID-19 vaccine booster for ages 12 to 15The FDA expanded Pfizer’s COVID-19 vaccine booster for kids as young as 12. Final approval from the CDC is still needed.
FDA expands Pfizer COVID-19 vaccine booster for ages 12 to 15The FDA expanded Pfizer’s COVID-19 vaccine booster for kids as young as 12. Final approval from the CDC is still needed.
Consulte Mais informação »
 U.S. FDA authorizes Pfizer's COVID-19 booster for 12- to 15-year-oldsThe U.S. Food and Drug Administration on Monday authorized the use of a third dose of the Pfizer and BioNTech COVID-19 vaccine for children ages 12 to 15, and narrowed the interval for booster shot eligibility to five months from six.
U.S. FDA authorizes Pfizer's COVID-19 booster for 12- to 15-year-oldsThe U.S. Food and Drug Administration on Monday authorized the use of a third dose of the Pfizer and BioNTech COVID-19 vaccine for children ages 12 to 15, and narrowed the interval for booster shot eligibility to five months from six.
Consulte Mais informação »
