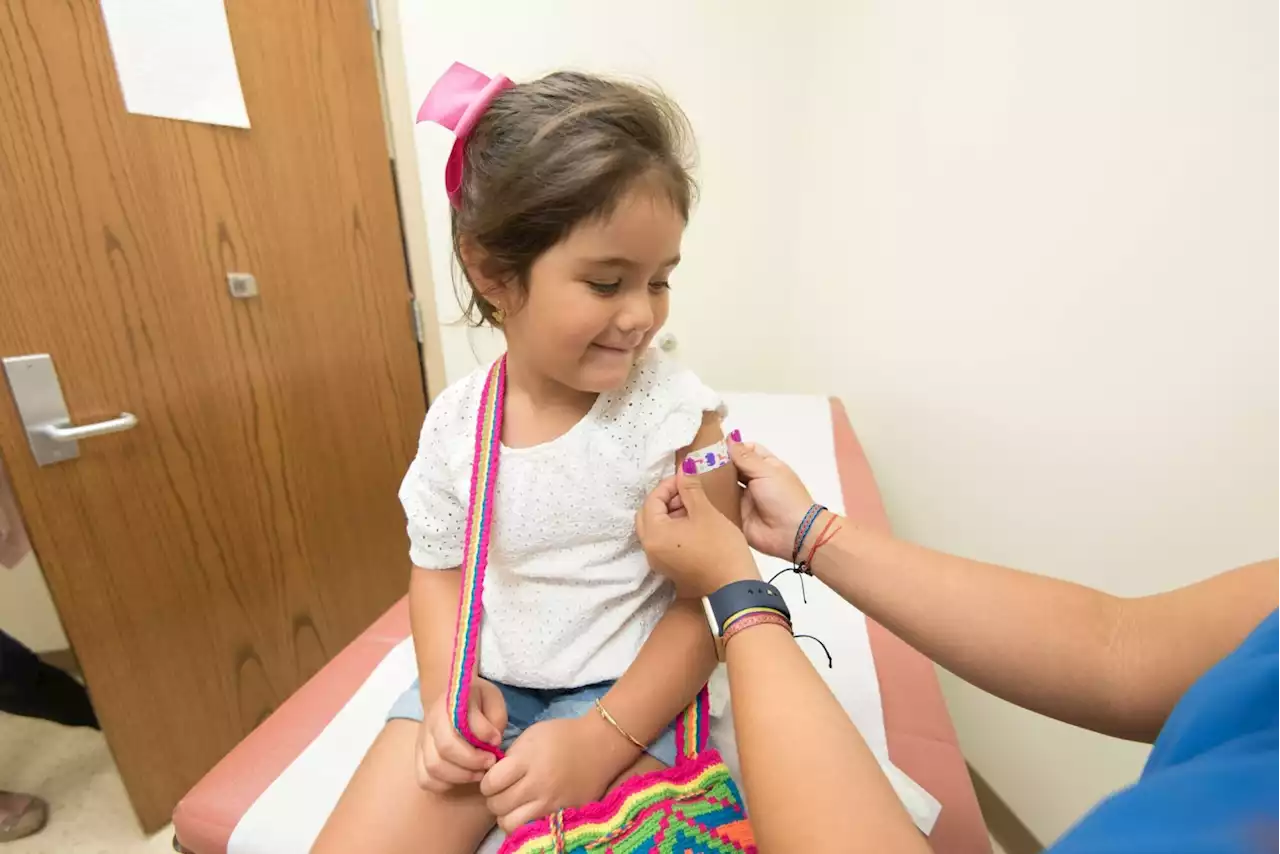
Vaccines for kids under 5 remain unapproved.
The US Food and Drug Administration has approved emergency use authorization of a Pfizer-BioNTech coronavirus booster shot for children ages 5 through 11.that any kid in that age group who finished their primary vaccination series at least five months ago can go ahead and get their booster. Pfizer-BioNTech’s mRNA-based vaccine is currently the only COVID-19 immunization and booster available for children ages 5 through 17.
“Vaccination continues to be the most effective way to prevent COVID-19 and its severe consequences, and it is safe,” FDA Commissioner Robert M. Califf said in a. “If your child is eligible for the Pfizer-BioNTech COVID-19 vaccine and has not yet received their primary series, getting them vaccinated can help protect them from the potentially severe consequences that can occur, such as hospitalization and death.
It’s unclear whether this new announcement will spur many parents to take their kids for COVID shots. Vaccination rates for children continue to lag far behind those of older populations.
Adults aged 18 and older in the US are eligible for primary vaccines and boosters made by Pfizer-BioNTech, Moderna, or Johnson & Johnson. People ages 50 and older are also eligible for a second booster, though the guidance for those boosters on the Centers for Disease Control and Prevention’s website was quietly updated Friday, indicating those who are eligiblein case variant-specific vaccines become available.
Another group must continue to wait, too: Parents of the country’s youngest children. The expected timelines for vaccine approval for children under 5 have been repeatedly pushed back. Both Pfizer and Moderna have been studying their vaccines’ effectiveness in small children, and the
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
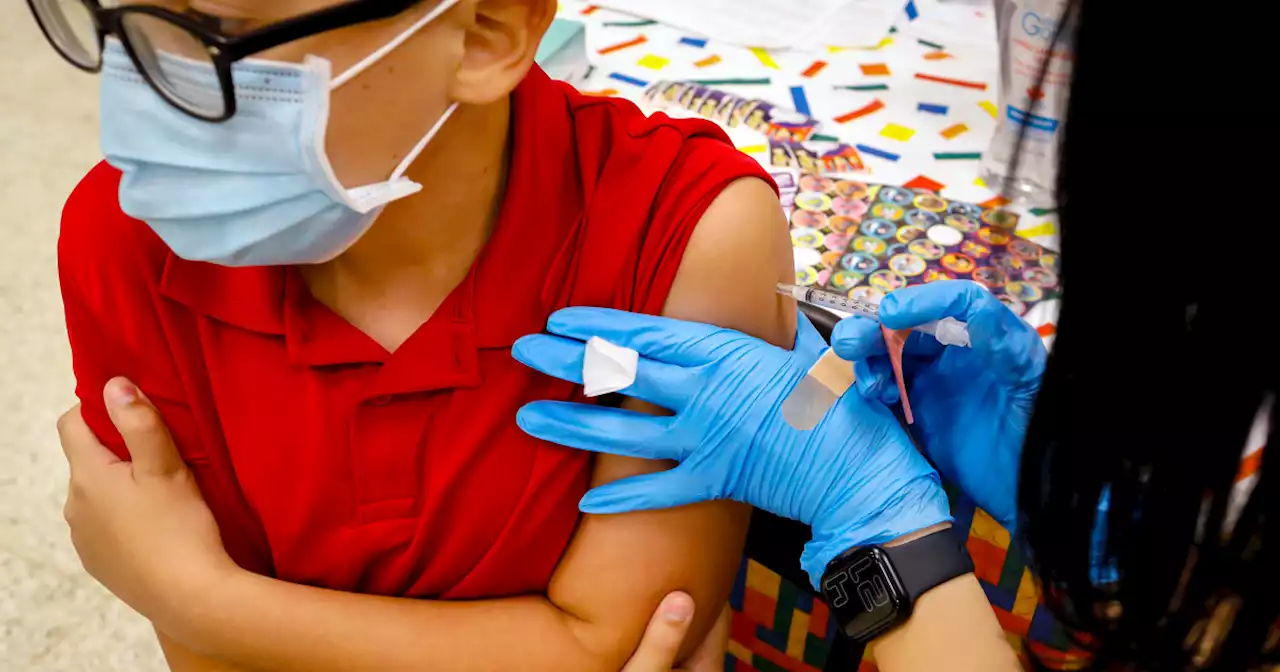 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Consulte Mais informação »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Consulte Mais informação »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Consulte Mais informação »
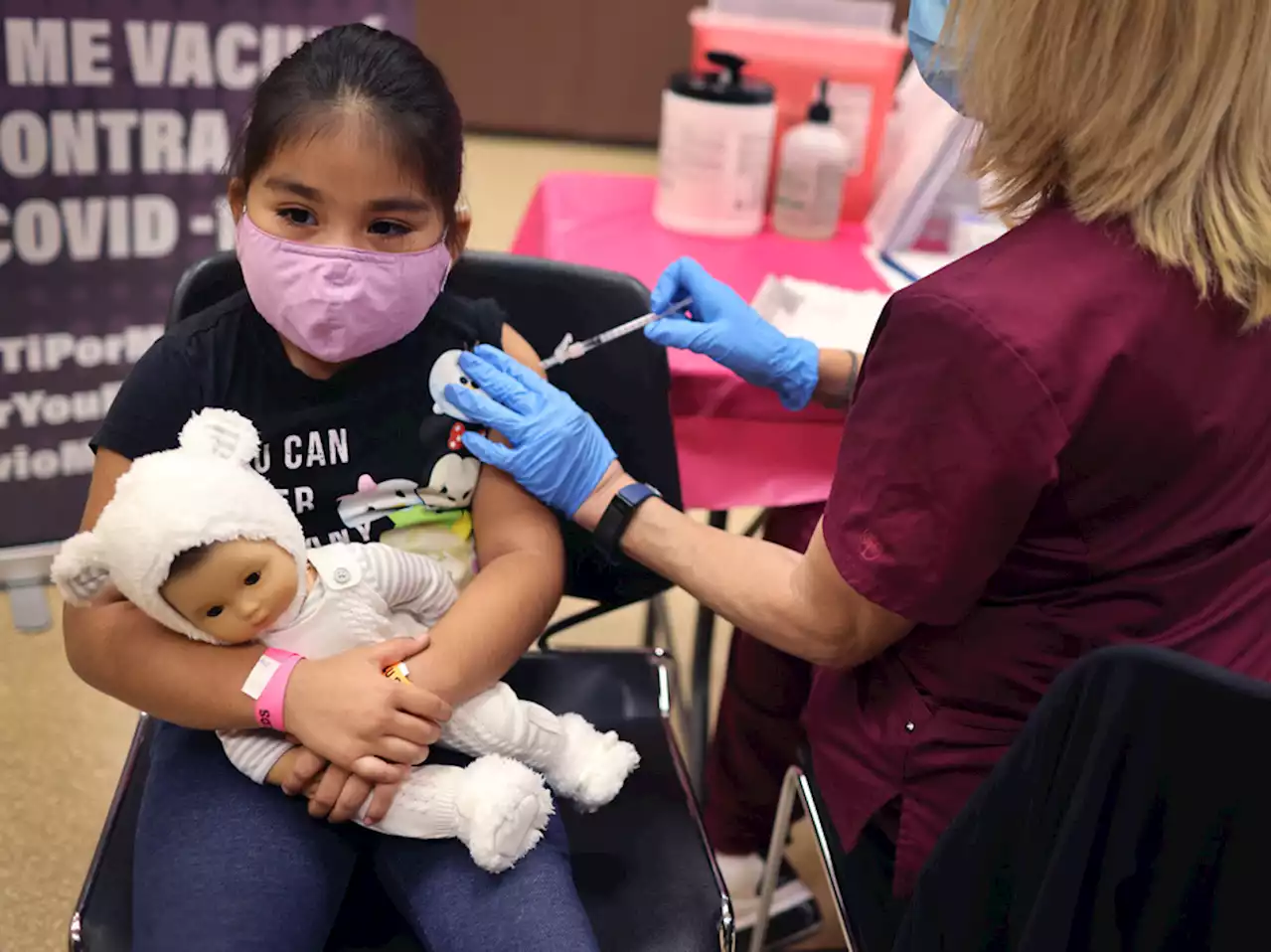 FDA authorizes first COVID booster for children ages 5 to 11The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
FDA authorizes first COVID booster for children ages 5 to 11The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
Consulte Mais informação »
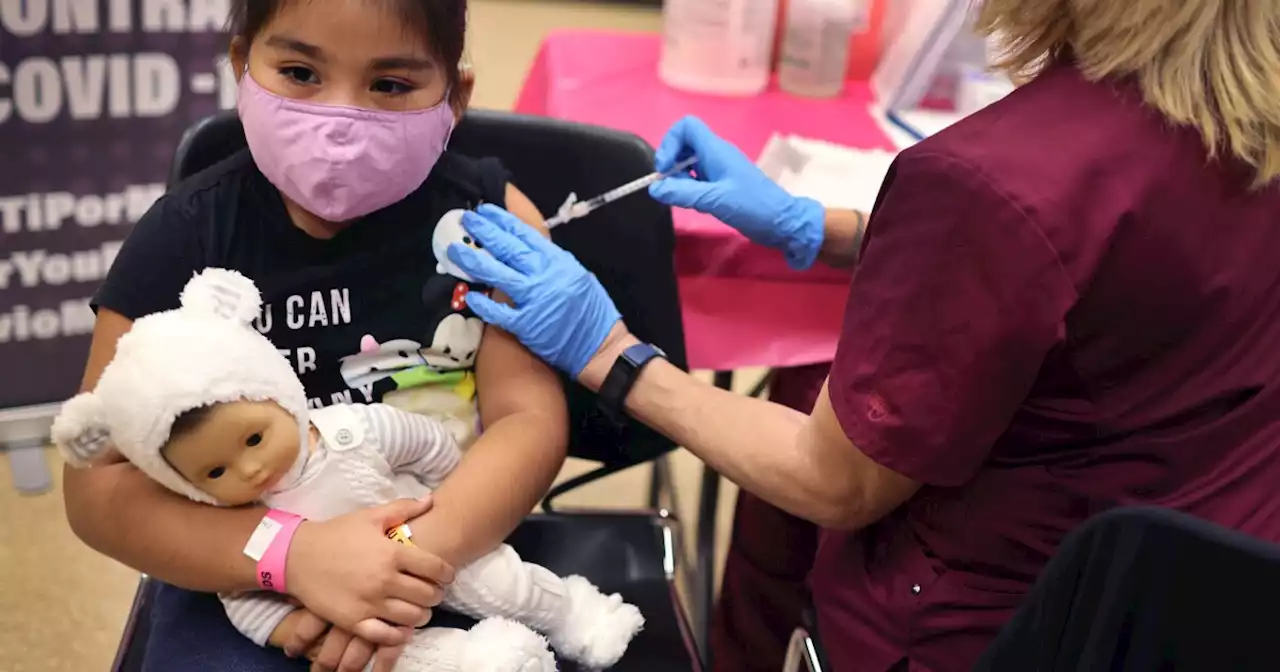 FDA authorizes first COVID booster for children ages 5 to 11The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
FDA authorizes first COVID booster for children ages 5 to 11The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
Consulte Mais informação »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
Consulte Mais informação »