The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
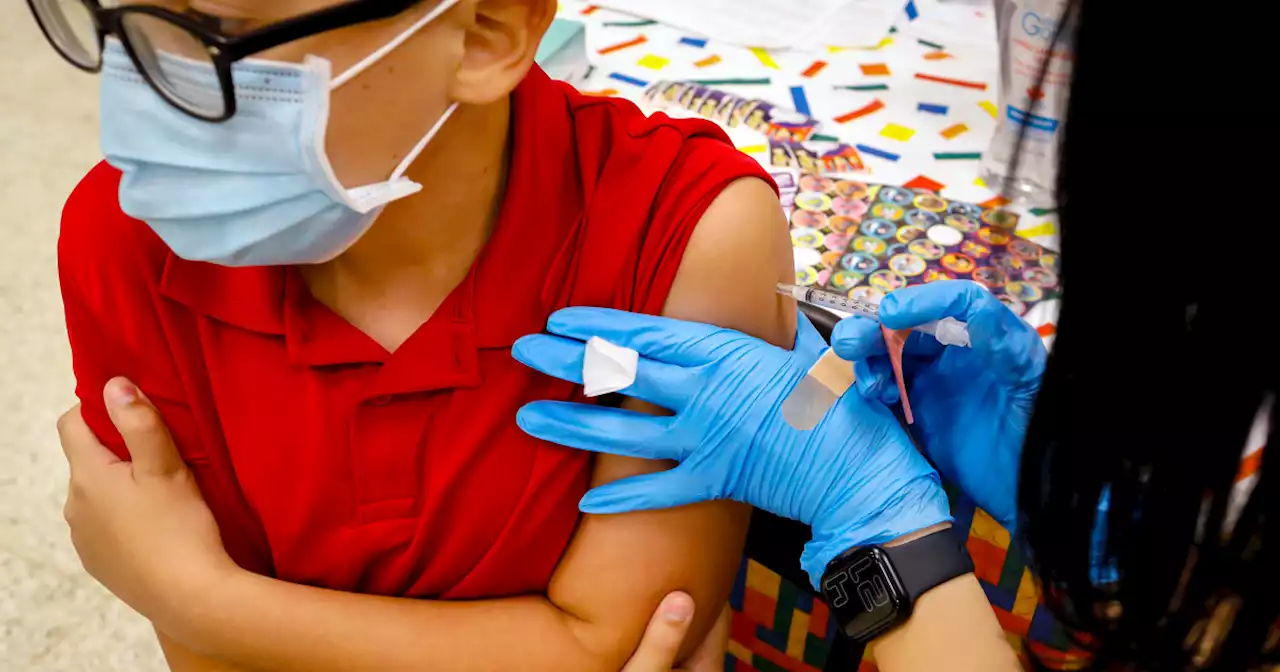
First grader Rihanna Chihuaque, 7, receives a COVID-19 vaccine at Arturo Velasquez Institute in Chicago last November.
The authorization makes all children in that age group who received their second shot at least five months ago eligible to receive a third shot of the Pfizer-BioNTech vaccine. "Since authorizing the vaccine for children down to 5 years of age in October 2021, emerging data suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine in all authorized populations," said Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, in a statement.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Consulte Mais informação »
 FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
Consulte Mais informação »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Consulte Mais informação »
 FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
Consulte Mais informação »
 FDA authorizes COVID-19 booster for children ages 5 to 11The Food and Drug Administration (FDA) amended Pfizer's COVID-19 booster shot emergency use authorization to allow for children ages 5 to 11 to receive the vaccine.
FDA authorizes COVID-19 booster for children ages 5 to 11The Food and Drug Administration (FDA) amended Pfizer's COVID-19 booster shot emergency use authorization to allow for children ages 5 to 11 to receive the vaccine.
Consulte Mais informação »
