A third pediatric dose of the Pfizer-BioNTech COVID-19 vaccine in children 6 months to under 5 years of age prompted a strong immune response, with a safety profile that was similar to placebo, the companies said:
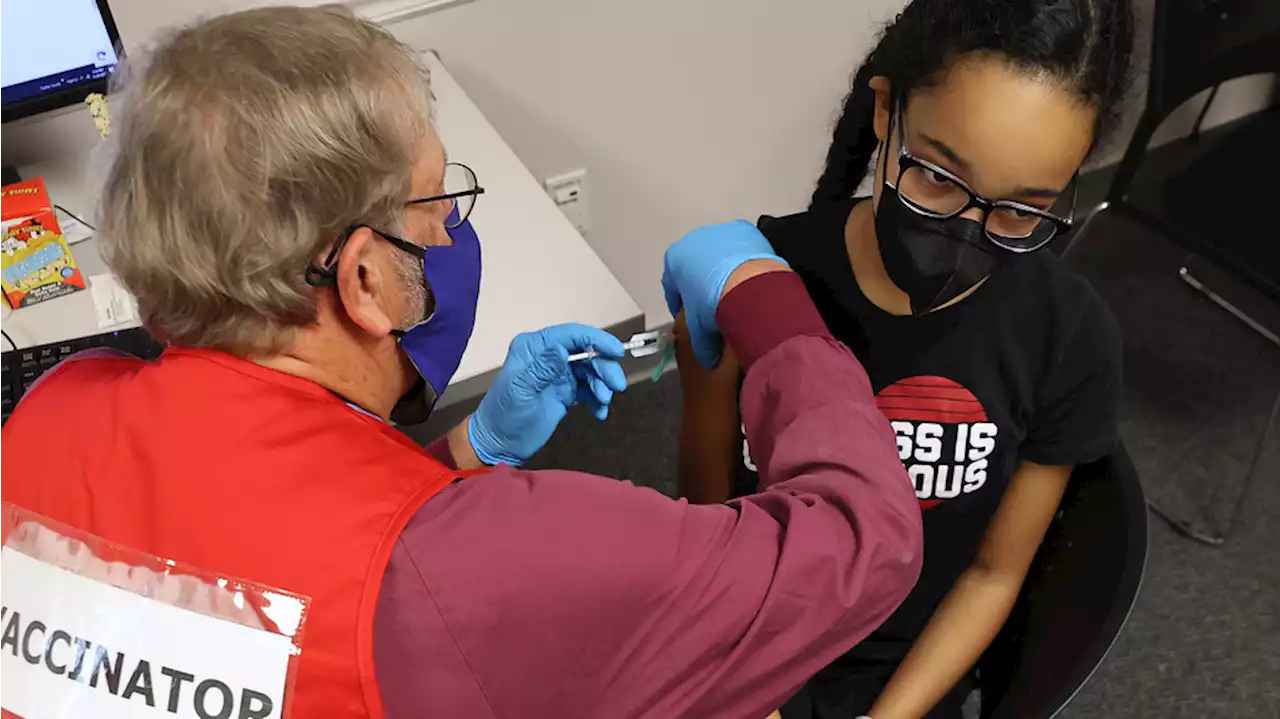
Pfizer will submit new data to the FDA this week about trials of its vaccine for kids younger than 5 years old. Here, an older child receives the Pfizer BioNTech COVID-19 vaccine in Virginia last November.
Pfizer's pediatric COVID-19 vaccine has an efficacy of 80.3%, according to a preliminary analysis, and meets"all immunobridging criteria required for Emergency Use Authorization," the company said Monday. The results are based on clinical trials in which kids from six months to age 5 got three doses of the company's vaccine.
Resumimos esta notícia para que você possa lê-la rapidamente. Se você se interessou pela notícia, pode ler o texto completo aqui. Consulte Mais informação: