The drug, Zavzpret, also known as zavegepant, was approved on Thursday for the treatment of acute migraines with or without an aura in adults.
“The FDA approval of Zavzpret marks a significant breakthrough for people with migraine who need freedom from pain and prefer alternative options to oral medications,” said Pfizer’s Angela Hwang, Chief Commercial Officer, President, Global Biopharmaceuticals Business.
The FDA’s greenlight hinged on late-stage study data, which showed Zavzpret was more effective than the placebo in 13 of 17 test categories, including providing pain relief in 15 minutes and restoring normal function at around half an hour. Among the 1,405 people who participated in the trial between October 2020 and August 2021, about 24% of those who took the drug said they experienced pain freedom two hours after treatment compared to 15% of people in the placebo group.
“When a migraine hits, it has a significant negative impact on a person’s daily life,” said Kathleen Mullin, M.D., Associate Medical Director at New England Institute for Neurology & Headache. “Among my migraine patients, one of the most important attributes of an acute treatment option is how quickly it works.”
A migraine is defined as a series of at least five headache attacks lasting between four and 72 hours, according to the National Headache Foundation. They can cause severe throbbing pain or a pulsing sensation in the head, with other symptoms often including nausea, vomiting, or sensitivity to light and sound.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA Approves Fast-Acting Nasal Spray for Migraines, Pfizer SaysClinical trials showed the nasal spray provided migraine relief within 15-30 minutes of use, with the relief lasting up to 48 hours in many patients.
FDA Approves Fast-Acting Nasal Spray for Migraines, Pfizer SaysClinical trials showed the nasal spray provided migraine relief within 15-30 minutes of use, with the relief lasting up to 48 hours in many patients.
Consulte Mais informação »
 The FDA Just Approved The First Fast-Acting Nasal Spray For MigrainesThe US Food and Drug Administration has approved a fast-acting nasal spray from Pfizer designed to treat migraines, the US pharmaceutical giant said Friday.
The FDA Just Approved The First Fast-Acting Nasal Spray For MigrainesThe US Food and Drug Administration has approved a fast-acting nasal spray from Pfizer designed to treat migraines, the US pharmaceutical giant said Friday.
Consulte Mais informação »
 FDA approves new nasal spray for migrainesMigraine sufferers will soon have something new to try to relieve their headaches fast.
FDA approves new nasal spray for migrainesMigraine sufferers will soon have something new to try to relieve their headaches fast.
Consulte Mais informação »
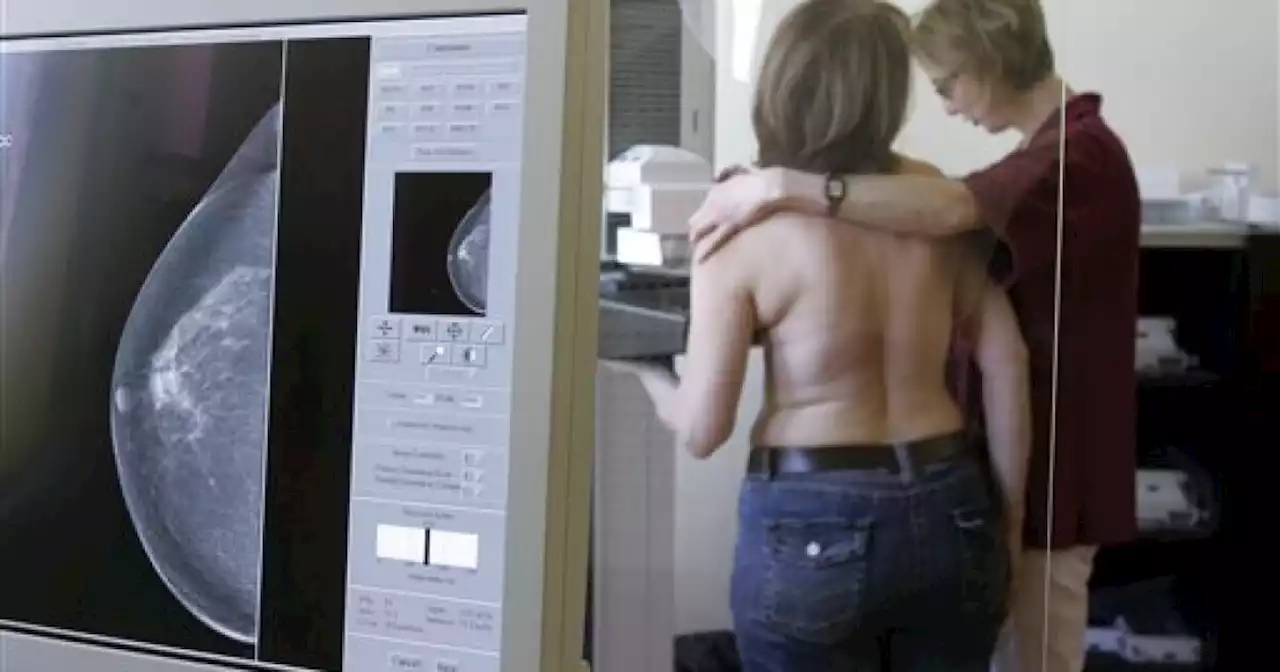 New FDA standards improve health, help strengthen the fight against breast cancerIn the next 18 months, the FDA will require mammogram facilities to let patients know if they have dense breast tissue so they can consult with a doctor if they need additional screening.
New FDA standards improve health, help strengthen the fight against breast cancerIn the next 18 months, the FDA will require mammogram facilities to let patients know if they have dense breast tissue so they can consult with a doctor if they need additional screening.
Consulte Mais informação »
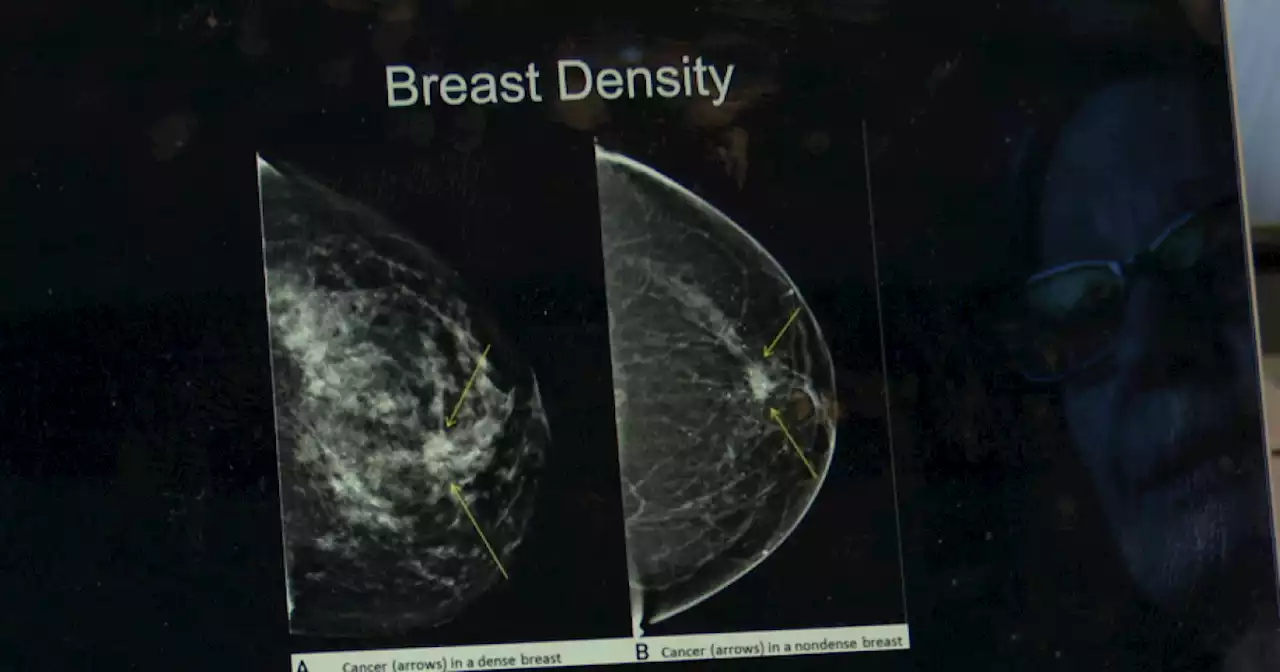 FDA updates mammogram guidelines in hopes to save livesDr. Foster recommends getting a mammogram once a year after you turn 40. About one in eight women will develop breast cancer in their life according to the CDC.
FDA updates mammogram guidelines in hopes to save livesDr. Foster recommends getting a mammogram once a year after you turn 40. About one in eight women will develop breast cancer in their life according to the CDC.
Consulte Mais informação »
 First FDA-cleared automated ear cleaner could become widely usedAn FDA-cleared automated ear cleaning system looks like a pair of headphones, and could become a widely used part of more automated healthcare.
First FDA-cleared automated ear cleaner could become widely usedAn FDA-cleared automated ear cleaning system looks like a pair of headphones, and could become a widely used part of more automated healthcare.
Consulte Mais informação »
