Pfizer Inc. undefined and BioNTech SE undefined said Monday that they asked the Food and Drug Administration to authorize their updated COVID-19 vaccine as...
Pfizer Inc. and BioNTech SE said Monday that they asked the Food and Drug Administration to authorize their updated COVID-19 vaccine as the third dose for children between the ages of 6 months and 5 years old. Children in this age group receive three doses as their primary series of shots.
PFE and BioNTech SE BNTX said Monday that they asked the Food and Drug Administration to authorize their updated COVID-19 vaccine as the third dose for children between the ages of 6 months and 5 years old. Children in this age group receive three doses as their primary series of shots.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Consulte Mais informação »
 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5.
Consulte Mais informação »
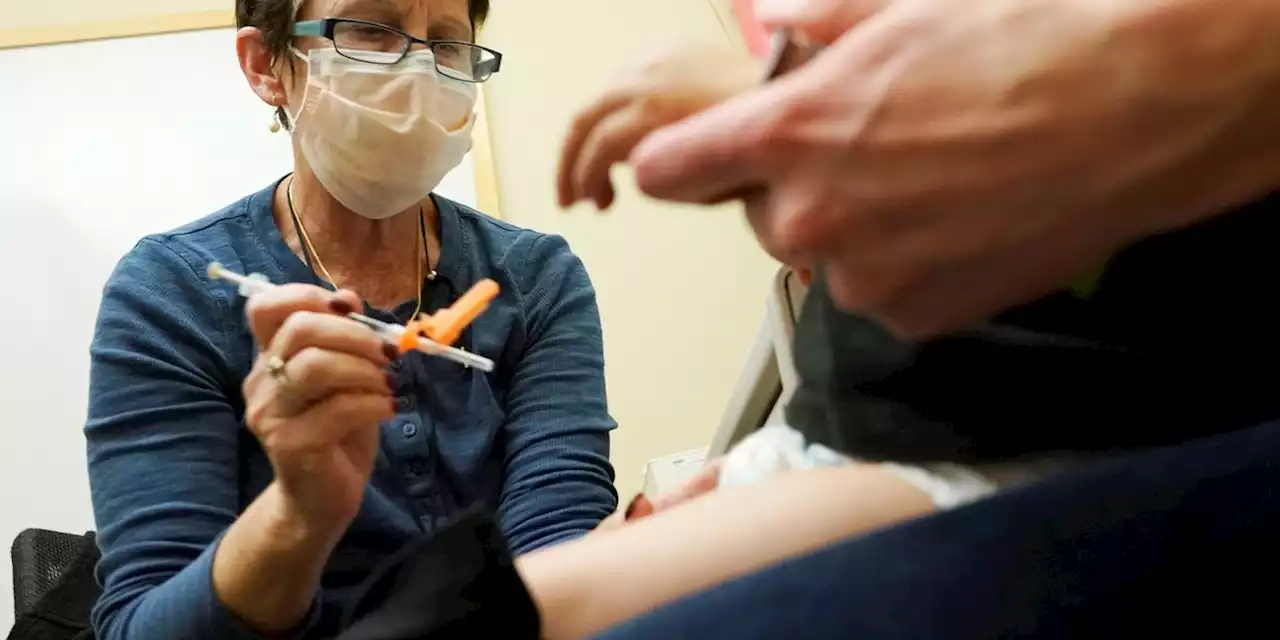 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Consulte Mais informação »
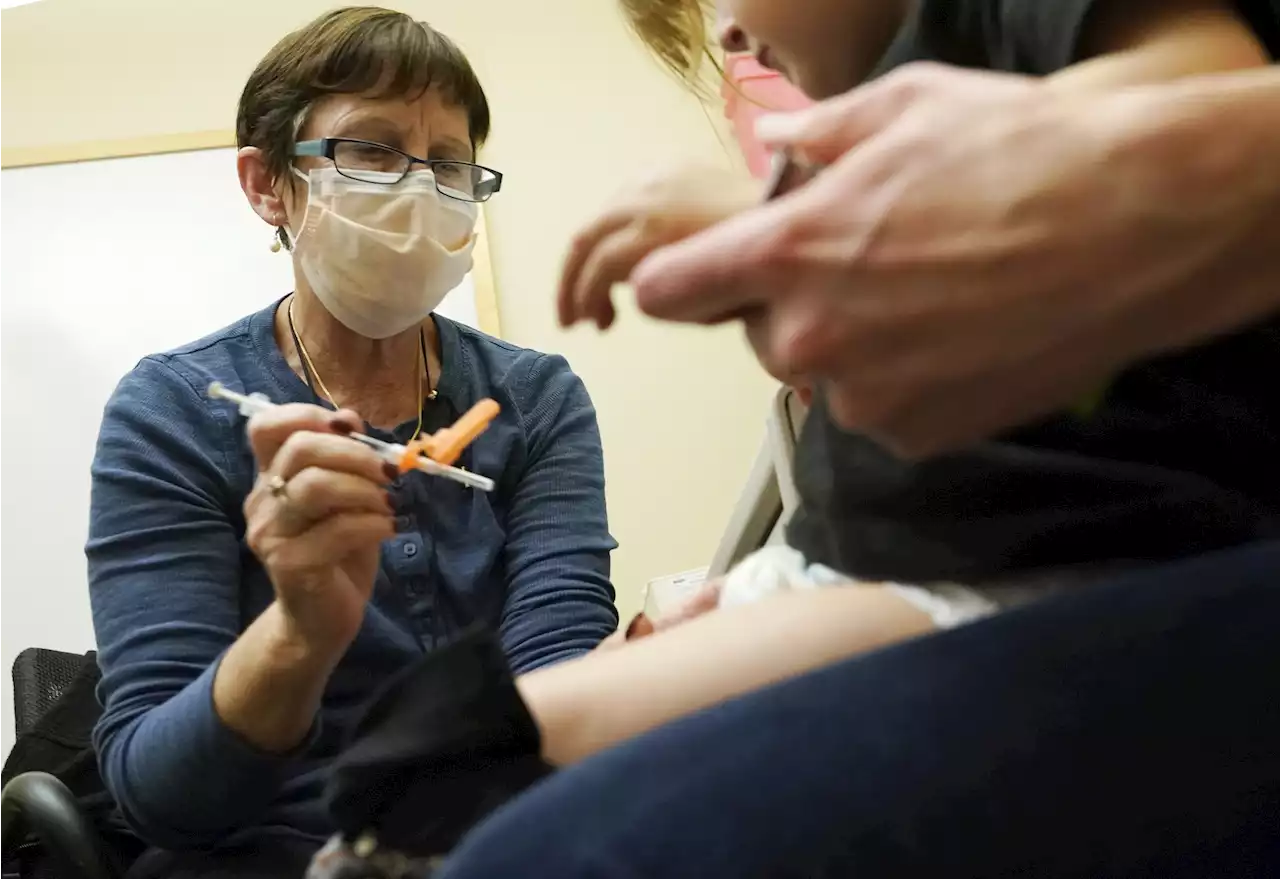 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series.
Consulte Mais informação »
 FDA Pulls US Authorization for Eli Lilly's COVID Drug BebtelovimabEli Lilly's COVID-19 drug bebtelovimab is not currently authorized for emergency use in the United States, the Food and Drug Administration said, citing it is not expected to neutralize the dominant BQ.1 and BQ.1.1 subvariants of Omicron.
FDA Pulls US Authorization for Eli Lilly's COVID Drug BebtelovimabEli Lilly's COVID-19 drug bebtelovimab is not currently authorized for emergency use in the United States, the Food and Drug Administration said, citing it is not expected to neutralize the dominant BQ.1 and BQ.1.1 subvariants of Omicron.
Consulte Mais informação »