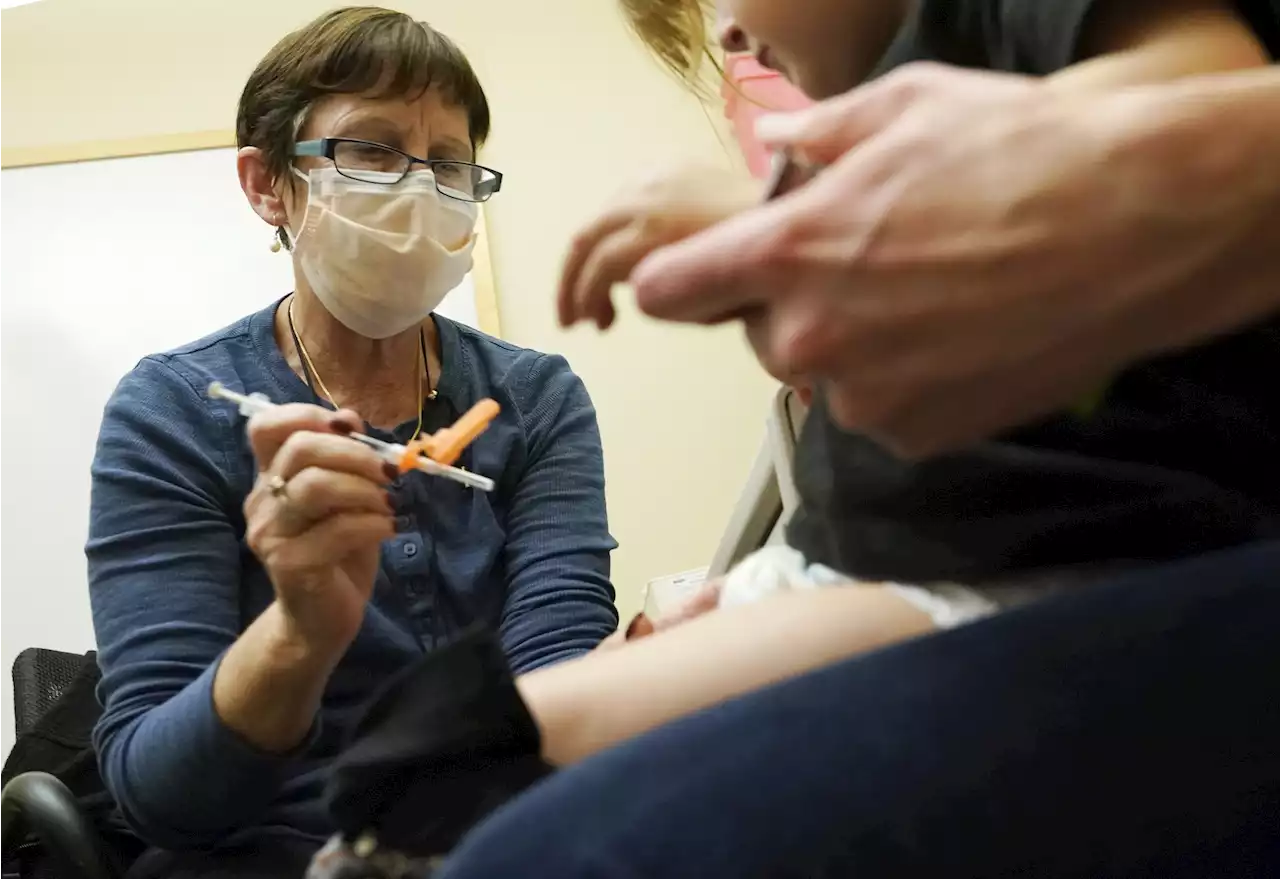
Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5, not as a booster but part of their initial series of three small-dose shots.
Deborah Sampson, left, a nurse at a University of Washington Medical Center clinic in Seattle, gives a Pfizer COVID-19 vaccine shot to a 20-month-old child, June 21, 2022, in Seattle. Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5. The youngest tots already are supposed to get three extra-small doses of the original vaccine as their primary series. Pfizer and its partner BioNTech said Monday, Dec.
Deborah Sampson, left, a nurse at a University of Washington Medical Center clinic in Seattle, gives a Pfizer COVID-19 vaccine shot to a 20-month-old child, June 21, 2022, in Seattle. Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5. The youngest tots already are supposed to get three extra-small doses of the original vaccine as their primary series. Pfizer and its partner BioNTech said Monday, Dec.
Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series. If the Food and Drug Administration agrees, a dose of Pfizer’s bivalent omicron-targeting vaccine would be substituted for their third shot.
Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.: Just 2% of tots under 2 and about 4% of 2- to 4-year-olds have gotten their primary doses so far, according to the Centers for Disease Control and Prevention.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Consulte Mais informação »
 FDA Pulls US Authorization for Eli Lilly's COVID Drug BebtelovimabEli Lilly's COVID-19 drug bebtelovimab is not currently authorized for emergency use in the United States, the Food and Drug Administration said, citing it is not expected to neutralize the dominant BQ.1 and BQ.1.1 subvariants of Omicron.
FDA Pulls US Authorization for Eli Lilly's COVID Drug BebtelovimabEli Lilly's COVID-19 drug bebtelovimab is not currently authorized for emergency use in the United States, the Food and Drug Administration said, citing it is not expected to neutralize the dominant BQ.1 and BQ.1.1 subvariants of Omicron.
Consulte Mais informação »
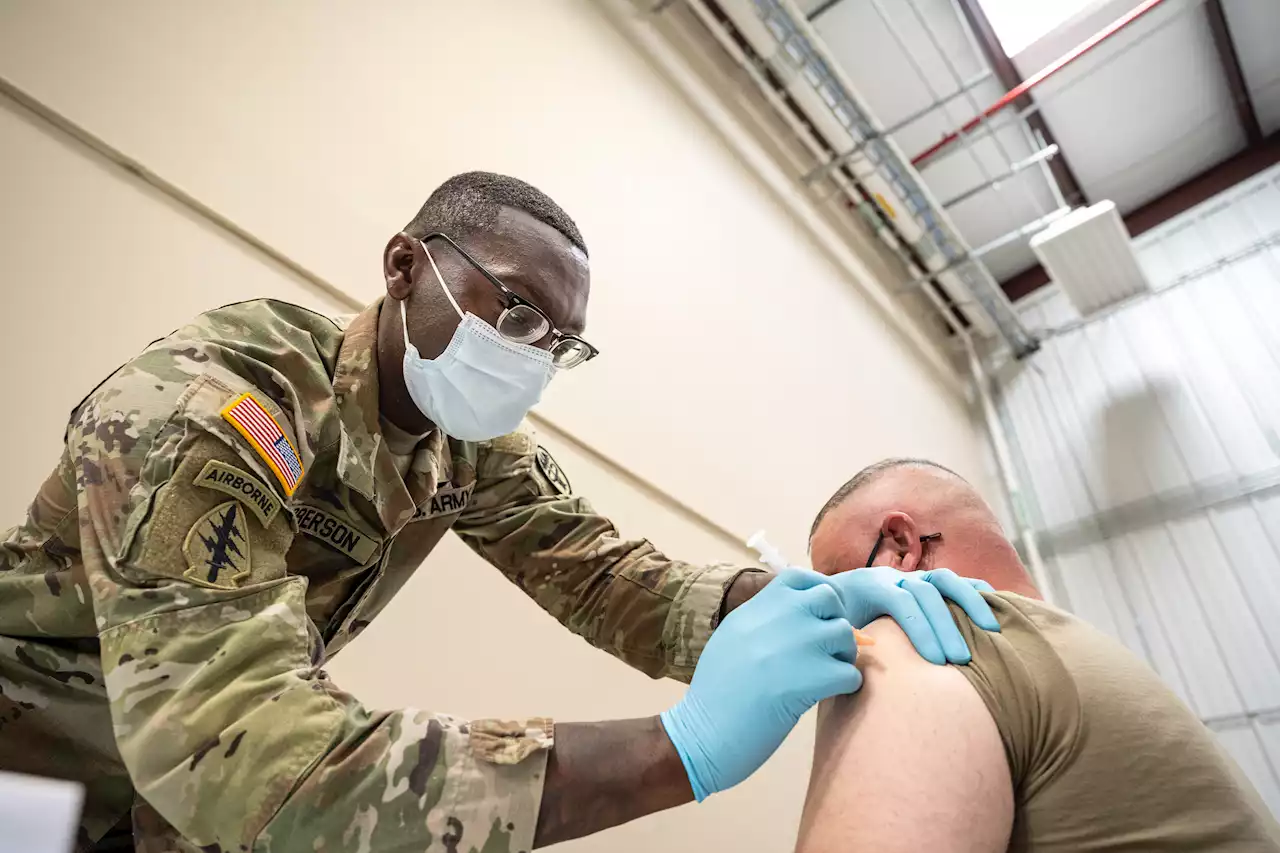 Defense bill could roll back Covid vaccine policy, top Dem saysThousands of troops have been kicked out for refusing the Covid vaccine. Undoing the 2021 policy would be a win for Republicans who argue it's exacerbating a recruiting and retention crisis.
Defense bill could roll back Covid vaccine policy, top Dem saysThousands of troops have been kicked out for refusing the Covid vaccine. Undoing the 2021 policy would be a win for Republicans who argue it's exacerbating a recruiting and retention crisis.
Consulte Mais informação »
 As COVID cases, other viruses continue to rise, SoCal inches closer to a mask mandateExperts say the combination of COVID, the flu, and RSV is putting an enormous strain on hospitals.
As COVID cases, other viruses continue to rise, SoCal inches closer to a mask mandateExperts say the combination of COVID, the flu, and RSV is putting an enormous strain on hospitals.
Consulte Mais informação »
 Covid hospitalizations rising post-Thanksgiving after an autumn lullA post-Thanksgiving uptick in covid-19 patients at U.S. hospitals is arriving even as health systems contend with waves of feverish, coughing people stricken with RSV and influenza infections.
Covid hospitalizations rising post-Thanksgiving after an autumn lullA post-Thanksgiving uptick in covid-19 patients at U.S. hospitals is arriving even as health systems contend with waves of feverish, coughing people stricken with RSV and influenza infections.
Consulte Mais informação »
 COVID-19 Monoclonal Antibody Treatments No Longer EffectiveThe FDA has said that bebtelovimab, a monoclonal antibody drug given through a vein, is no longer authorized because it is not effective against the leading strains of COVID-19. This drug was the last remaining monoclonal antibody treatment of six that had been authorized for emergency use during the pandemic.
COVID-19 Monoclonal Antibody Treatments No Longer EffectiveThe FDA has said that bebtelovimab, a monoclonal antibody drug given through a vein, is no longer authorized because it is not effective against the leading strains of COVID-19. This drug was the last remaining monoclonal antibody treatment of six that had been authorized for emergency use during the pandemic.
Consulte Mais informação »