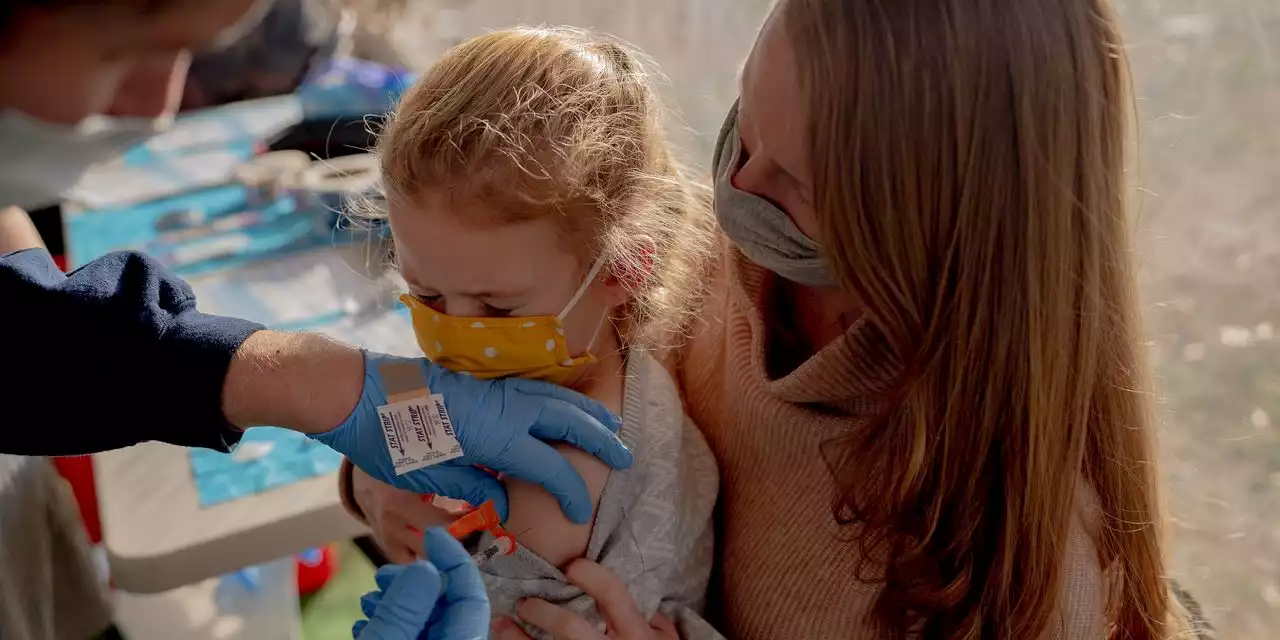
Pfizer has asked the FDA to authorize a booster dose of its Covid-19 vaccine for children 5 to 11 years old
Pfizer and partner BioNTech made the request after testing found the extra dose produced a strong immune response and was safe
Children are less likely to become infected with Covid-19; a 6-year-old girl received a Covid-19 vaccine in December in Stamford, Conn.Pfizer Inc. and partner BioNTech SE asked U.S. health regulators to authorizeThe request Tuesday to the Food and Drug Administration comes after the companies said earlier this month that a third shot safely generated a strong immune response in the youngsters, including significantly increased antibody levels against the Omicron variant.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Biden announces efforts to improve access to Pfizer COVID-19 treatmentThe Biden administration said it will attempt to save more lives with Pfizer’s effective treatment for COVID-19 by nearly doubling the number of pharmacies that stock the pills and setting up federally supported “test-to-treat” sites that offer the drug to people who test positive.
Biden announces efforts to improve access to Pfizer COVID-19 treatmentThe Biden administration said it will attempt to save more lives with Pfizer’s effective treatment for COVID-19 by nearly doubling the number of pharmacies that stock the pills and setting up federally supported “test-to-treat” sites that offer the drug to people who test positive.
Consulte Mais informação »
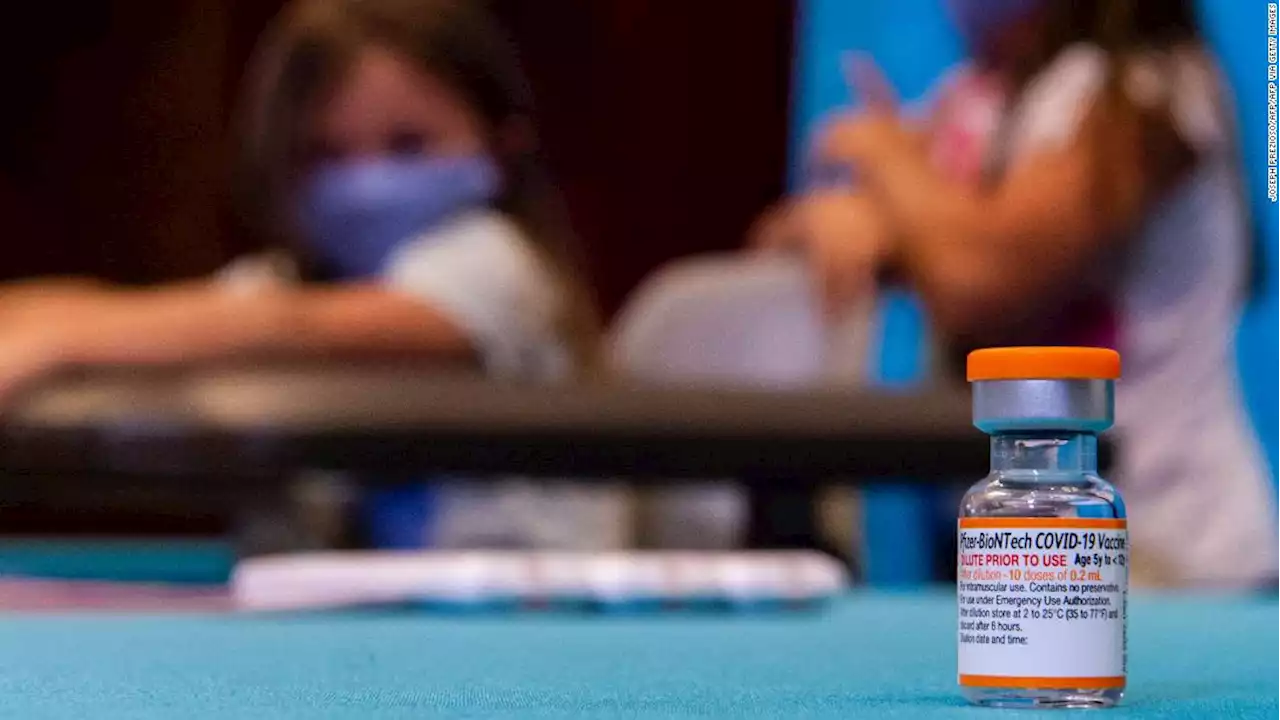 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Consulte Mais informação »
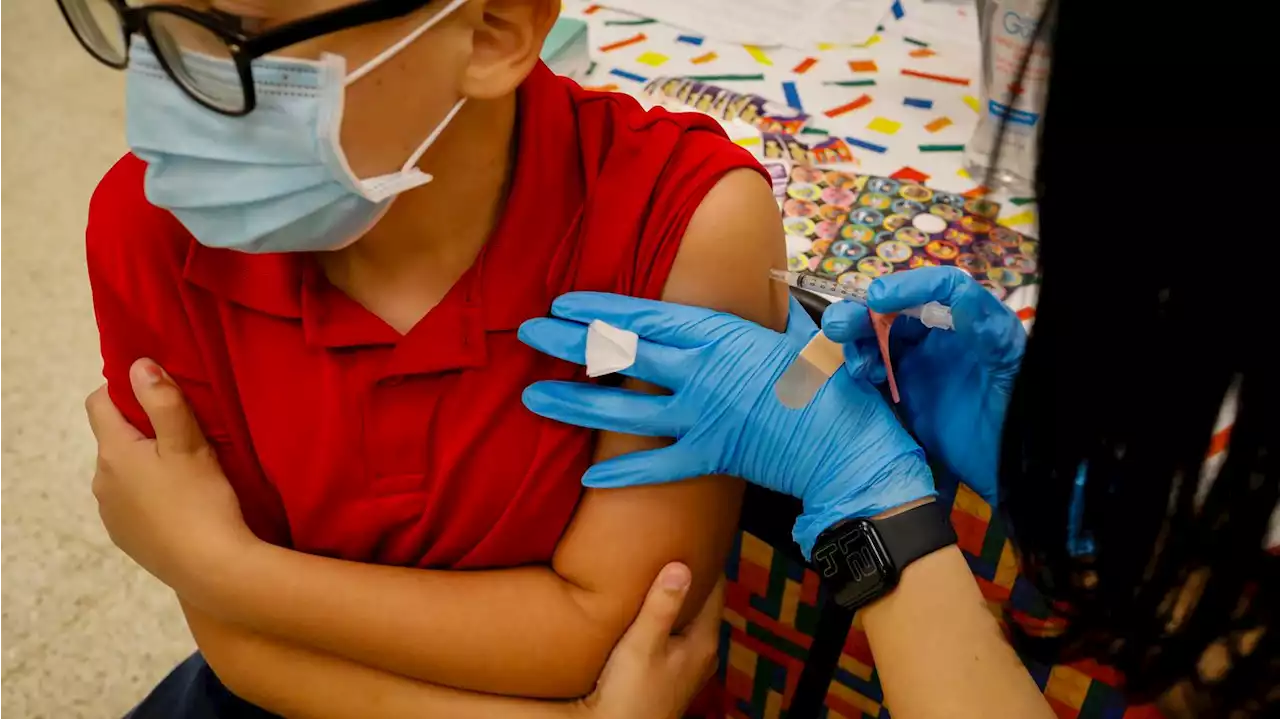 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Consulte Mais informação »
 FDA approves COVID drug for children, China deals with uphill COVID battle, and more COVID newsThe US Food and Drug Administration announced Monday that it has expanded approval of the COVID-19 drug remdesivir to treat patients as young as 28 days old. Here's that and more COVID news.
FDA approves COVID drug for children, China deals with uphill COVID battle, and more COVID newsThe US Food and Drug Administration announced Monday that it has expanded approval of the COVID-19 drug remdesivir to treat patients as young as 28 days old. Here's that and more COVID news.
Consulte Mais informação »
 FDA approves first COVID-19 treatment for children under 12 years oldThe FDA gave approval to the use of Veklury (remdesivir) for children 28 days and older who weigh at least 3 kilograms (about 7 pounds).
FDA approves first COVID-19 treatment for children under 12 years oldThe FDA gave approval to the use of Veklury (remdesivir) for children 28 days and older who weigh at least 3 kilograms (about 7 pounds).
Consulte Mais informação »
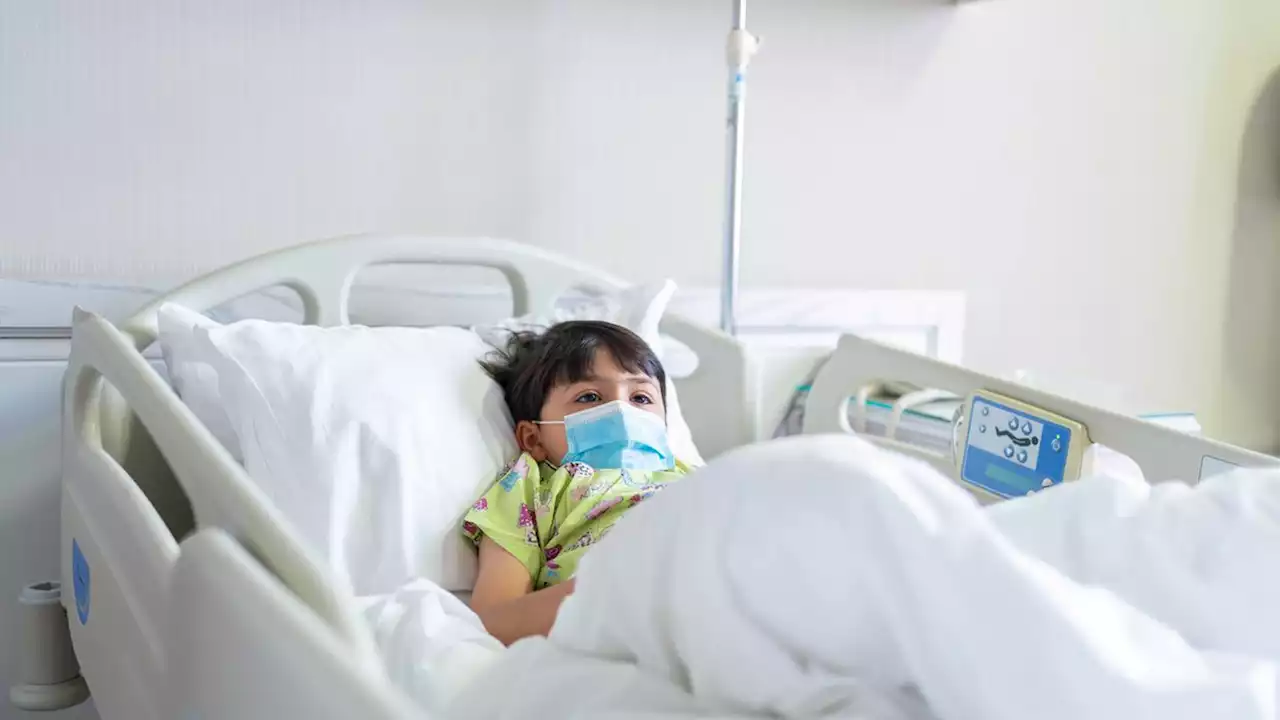 Coronavirus: FDA approves first COVID-19 treatment for children younger than 12The FDA said Monday it expanded the approval of Veklury to include pediatric patients in certain circumstances.
Coronavirus: FDA approves first COVID-19 treatment for children younger than 12The FDA said Monday it expanded the approval of Veklury to include pediatric patients in certain circumstances.
Consulte Mais informação »