Children under the age of 5 are the only group not yet eligible for vaccination — but that could change soon.
New Orleans became the first major school district to require its students ages 5 and up to be vaccinated against COVID. Parents can opt out on medical, religious or philosophical grounds.
But with so many parents already hesitant to vaccinate their eligible children, experts don't expect authorization for kids under 5 to significantly affect new case numbers. Only 38% of 5- to 17-year-olds are fully vaccinated. "I encourage parents to talk to their pediatricians, to read trustworthy sources of information, not to go down social media rabbit holes, but to look at the real data," Dr. Megan Ranney, an emergency physician and academic dean of public health at Brown University, told CBS News. "The second it is available, we're going to be calling our pediatrician," Preti told CBS News."I feel very comfortable with having my kids get the vaccine.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Pfizer submits request for COVID-19 vaccine use in children under age 5Pfizer and BioNTech said Tuesday that the companies had asked the Food and Drug Administration (FDA) to authorize their COVID-19 vaccine for emergency use in children younger than 5 years old.
Pfizer submits request for COVID-19 vaccine use in children under age 5Pfizer and BioNTech said Tuesday that the companies had asked the Food and Drug Administration (FDA) to authorize their COVID-19 vaccine for emergency use in children younger than 5 years old.
Consulte Mais informação »
 Pfizer asks FDA to allow COVID-19 vaccine for kids under 5Pfizer on Tuesday asked the U.S. to authorize extra-low doses of its COVID-19 vaccine for children under 5, potentially opening the way for the very youngest Americans to start receiving shots as early as March.
Pfizer asks FDA to allow COVID-19 vaccine for kids under 5Pfizer on Tuesday asked the U.S. to authorize extra-low doses of its COVID-19 vaccine for children under 5, potentially opening the way for the very youngest Americans to start receiving shots as early as March.
Consulte Mais informação »
 Regulators urge Pfizer to apply for COVID-19 vaccines for kids under 5Early Pfizer data has shown the vaccine — which is administered to younger kids at one-tenth the strength of the adult shot — is safe and produces an immune response.
Regulators urge Pfizer to apply for COVID-19 vaccines for kids under 5Early Pfizer data has shown the vaccine — which is administered to younger kids at one-tenth the strength of the adult shot — is safe and produces an immune response.
Consulte Mais informação »
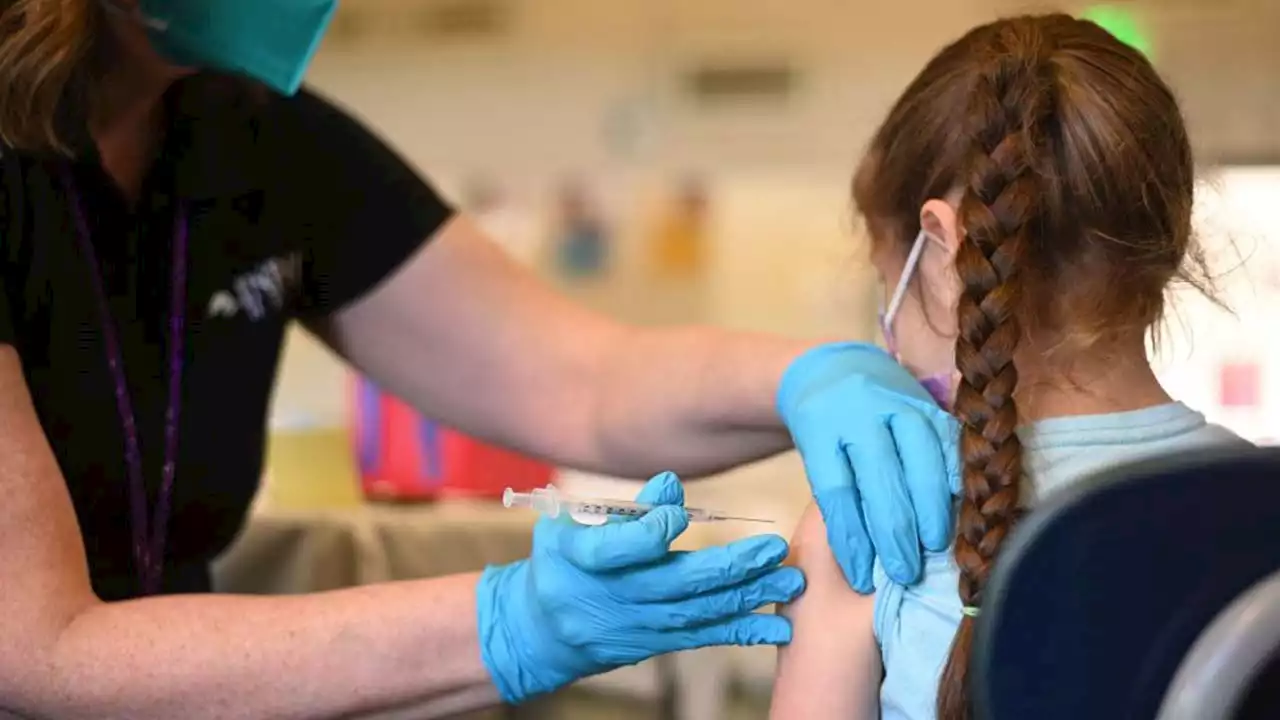 Pfizer asks FDA for emergency approval of COVID-19 vaccine for kids under 5VACCINE FOR KIDS: Children 6 months to 5 years old are one step closer to receiving the Pfizer COVID-19 vaccine after the company officially submitted the two-dose regimen to the FDA for emergency approval.
Pfizer asks FDA for emergency approval of COVID-19 vaccine for kids under 5VACCINE FOR KIDS: Children 6 months to 5 years old are one step closer to receiving the Pfizer COVID-19 vaccine after the company officially submitted the two-dose regimen to the FDA for emergency approval.
Consulte Mais informação »
