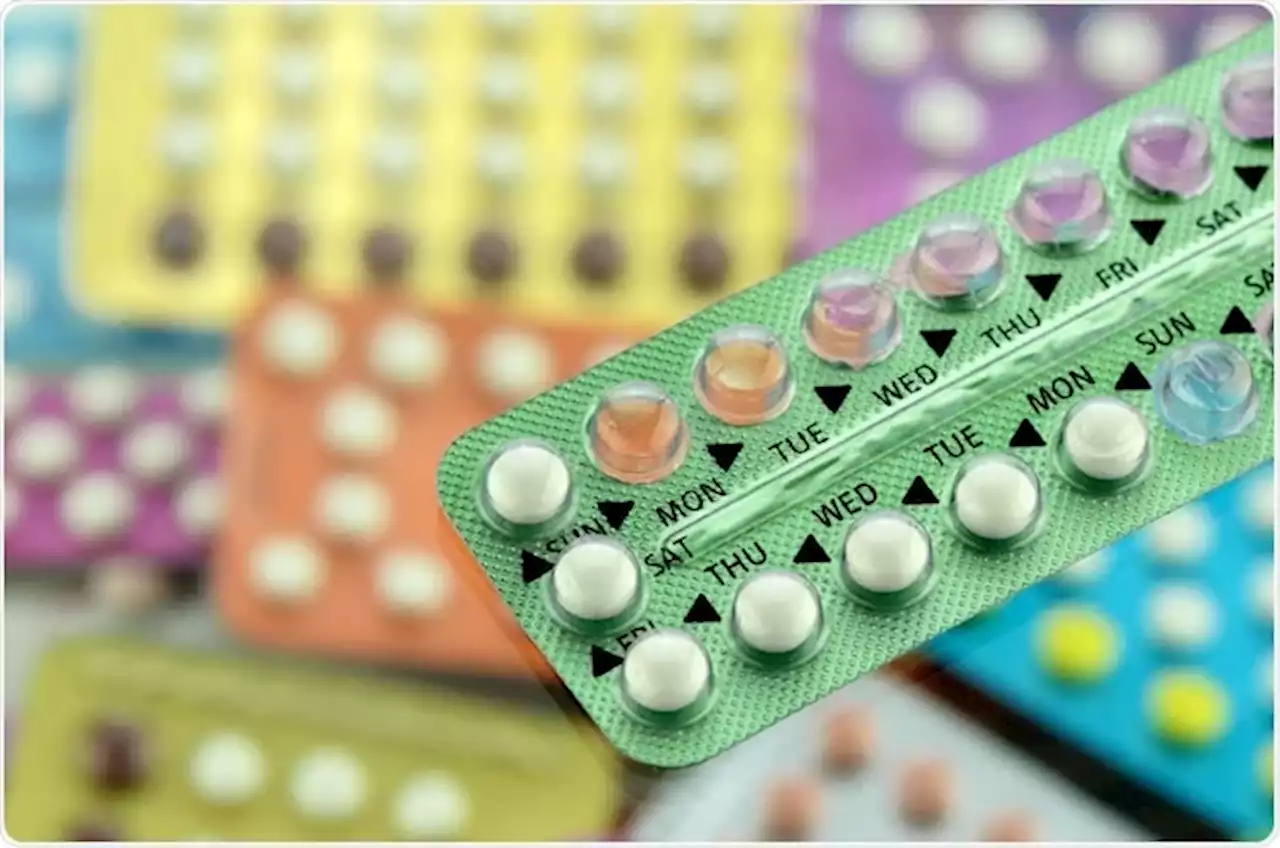
On Wednesday, a panel of FDA advisors unanimously voted to recommend the FDA approve the first over-the-counter birth control pill in the United States.
The FDA approved the birth control pill for distribution in the United States by prescription in 1960, but it is currently only available with a prescription from a doctor or pharmacist. recommend the FDA approve the first over-the-counter birth control pill
The decision does not mean the pill is available over the counter—yet. But the decision from the influential advisory panel marks a significant step toward making birth control more widely available. Opill, which is made of progestin, is not recommended for people who have had breast cancer. FDA scientists said their reservations about making the progestin-only birth control pill available without a prescription are whether women with medical conditions that should preclude them from taking the pill—mainly, breast cancer and undiagnosed vaginal bleeding—would self-select and avoid the product.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
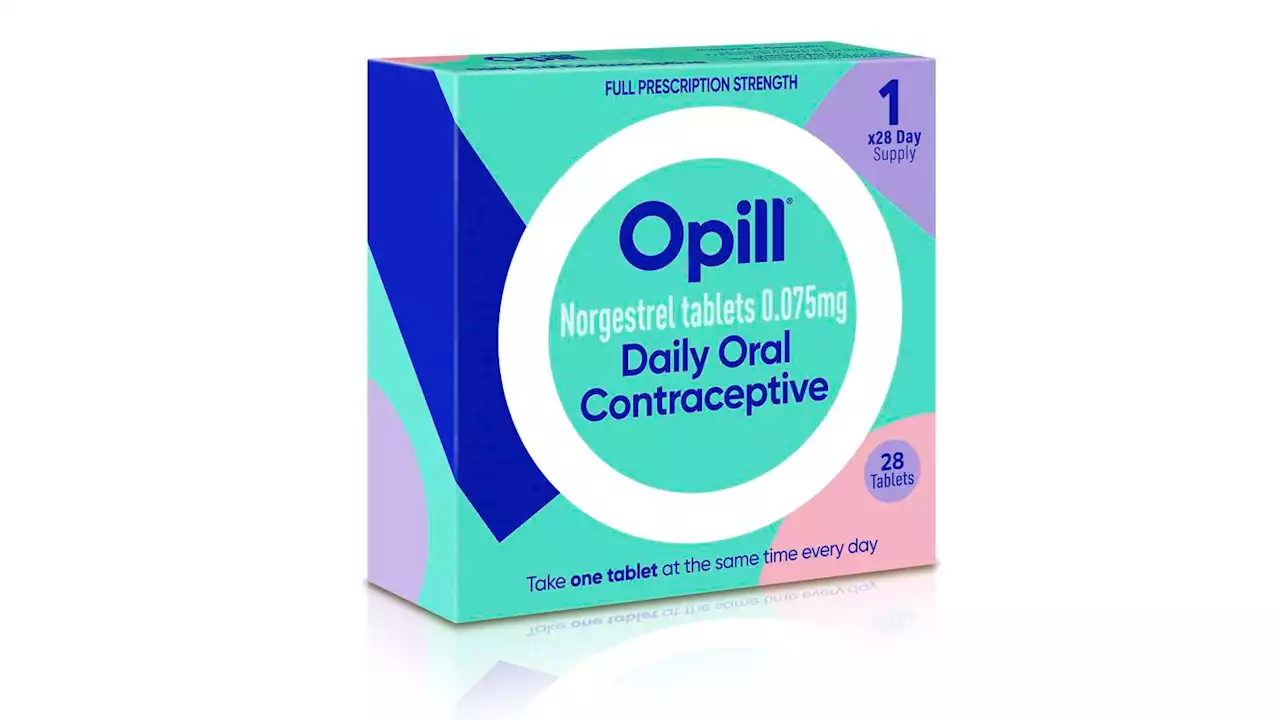 FDA panel backs over-the-counter birth control pillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription
FDA panel backs over-the-counter birth control pillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription
Consulte Mais informação »
 FDA panel backs over-the-counter birth control pillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription. The positive vote on Wednesday by a Food and Drug Administration panel paves the way for what could be the first birth control pill available over the counter. The recommendation is not binding and the FDA is expected to make its decision on the drug this summer. Currently all contraceptive pills in the U.S. require a prescription. Dozens of medical and advocacy groups support making the pill available without a prescription to increase birth control options for women.
FDA panel backs over-the-counter birth control pillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription. The positive vote on Wednesday by a Food and Drug Administration panel paves the way for what could be the first birth control pill available over the counter. The recommendation is not binding and the FDA is expected to make its decision on the drug this summer. Currently all contraceptive pills in the U.S. require a prescription. Dozens of medical and advocacy groups support making the pill available without a prescription to increase birth control options for women.
Consulte Mais informação »
 FDA Panel Backs Over-The-Counter Birth Control PillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription.
FDA Panel Backs Over-The-Counter Birth Control PillFederal health advisers are recommending that a decades-old birth control pill be sold without a prescription.
Consulte Mais informação »
 FDA panel backs over-the-counter birth control pillU.S. Food and Drug Administration advisers endorsed the first over-the-counter sales of a birth control pill, with a final decision expected this summer.
FDA panel backs over-the-counter birth control pillU.S. Food and Drug Administration advisers endorsed the first over-the-counter sales of a birth control pill, with a final decision expected this summer.
Consulte Mais informação »
 FDA panel backs over-the-counter birth control pill, teeing up approvalBREAKING: An FDA panel voted to back the sale of an over-the-counter birth control pill, clearing the way for approval.
FDA panel backs over-the-counter birth control pill, teeing up approvalBREAKING: An FDA panel voted to back the sale of an over-the-counter birth control pill, clearing the way for approval.
Consulte Mais informação »
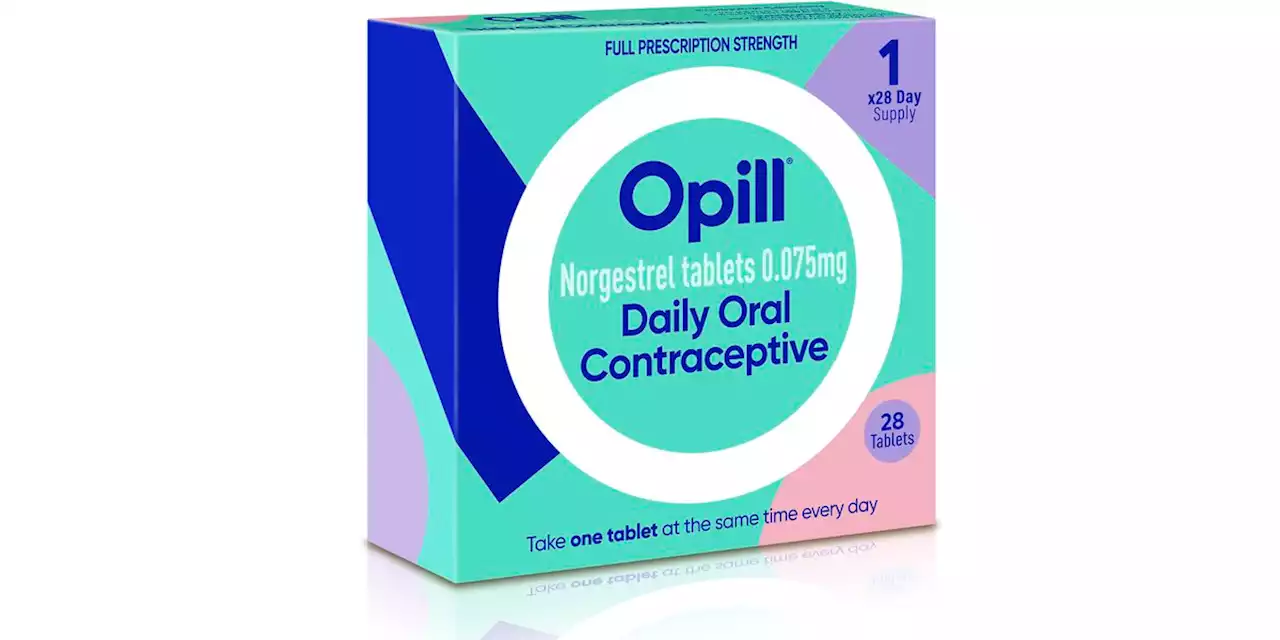 FDA panel backs over-the-counter birth control pillIf the agency follows the nonbinding recommendation, Perrigo’s drug, Opill, would become the first contraceptive pill to be moved out from behind the pharmacy counter onto store shelves.
FDA panel backs over-the-counter birth control pillIf the agency follows the nonbinding recommendation, Perrigo’s drug, Opill, would become the first contraceptive pill to be moved out from behind the pharmacy counter onto store shelves.
Consulte Mais informação »