U.S. health regulators on Friday approved a new type of drug for women dealing with uncomfortable hot flashes caused by menopause.
In this undated product photo released by Astellas Pharma, a box and container of Veozah drug are displayed. –
Astellas' drug, Veozah, uses a new approach, targeting brain connections that help control body temperature. The FDA said the medication will provide"an additional safe and effective treatment option for women,” in a statement. The most common treatment consists of hormonal pills aimed at boosting levels of estrogen and progestin. But the treatment isn't appropriate for some women, including those with a history of stroke, blood clots, heart attack and other health conditions. Large studies have found that the hormones can increase the chances of those problems reoccurring, although the risks vary based on a number of individual factors.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
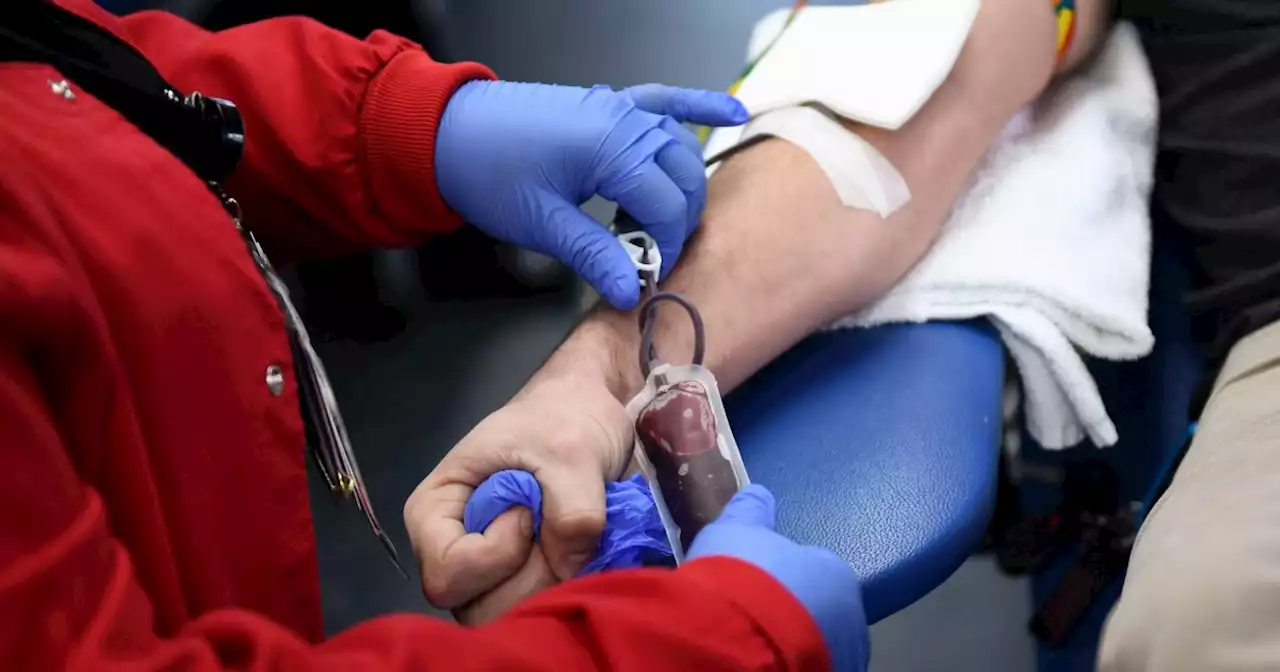 More gay and bisexual men can donate blood under new FDA rulesMost gay and bisexual men who are in a monogamous relationship with another man will no longer need to abstain from sex to donate blood.
More gay and bisexual men can donate blood under new FDA rulesMost gay and bisexual men who are in a monogamous relationship with another man will no longer need to abstain from sex to donate blood.
Consulte Mais informação »
 FDA finalizes new rules on blood donation for gay and bisexual menThe step paves the way for more blood donors and represents another step in ending a discriminatory and outdated policy.
FDA finalizes new rules on blood donation for gay and bisexual menThe step paves the way for more blood donors and represents another step in ending a discriminatory and outdated policy.
Consulte Mais informação »
 New FDA Rule Allows More Gay and Bisexual Men to Donate BloodGay and bisexual men were previously subject to a lifetime ban on blood donations, which was widely regarded as discriminatory.
New FDA Rule Allows More Gay and Bisexual Men to Donate BloodGay and bisexual men were previously subject to a lifetime ban on blood donations, which was widely regarded as discriminatory.
Consulte Mais informação »
 FDA OKs New Drug for Fabry DiseasePegunigalsidase alfa (Elfabrio) is an intravenously administered enzyme replacement therapy to treat adults with confirmed Fabry disease.
FDA OKs New Drug for Fabry DiseasePegunigalsidase alfa (Elfabrio) is an intravenously administered enzyme replacement therapy to treat adults with confirmed Fabry disease.
Consulte Mais informação »
 New FDA rules allow more gay, bisexual men to donate bloodGay rights groups have long opposed blanket restrictions on who can give blood, saying they discriminate. Medical societies including the American Medical Association have also said such exclusions…
New FDA rules allow more gay, bisexual men to donate bloodGay rights groups have long opposed blanket restrictions on who can give blood, saying they discriminate. Medical societies including the American Medical Association have also said such exclusions…
Consulte Mais informação »
 Experts react to FDA’s new guidelines on blood donations from gay, bisexual menNew guidelines from the U.S. Food and Drug Administration will let more gay and bisexual men donate blood.
Experts react to FDA’s new guidelines on blood donations from gay, bisexual menNew guidelines from the U.S. Food and Drug Administration will let more gay and bisexual men donate blood.
Consulte Mais informação »
