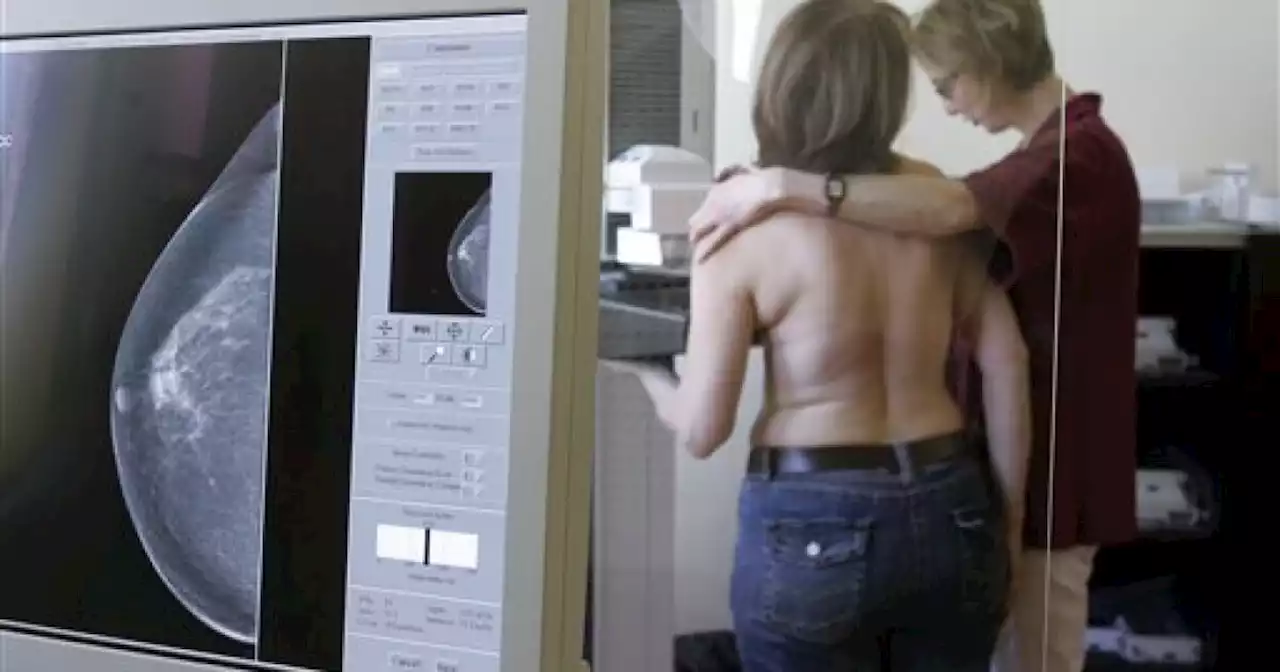
In the next 18 months, the FDA will require mammogram facilities to let patients know if they have dense breast tissue so they can consult with a doctor if they need additional screening.
CLEVELAND — As one of many living in the United States with dense breasts, Kimberly Johnson says she has to go through more additional breast cancer screening steps than the average person since doctors say her density makes it more challenging to detect breast cancer with a regular mammogram.
That’s why Johnson says she supports a new nationwide push from the FDA to require mammogram facilities to let patients know if they have dense breast tissue so they can consult with a doctor if they need additional screening. Marshall says only 38 states require some sort of reporting about breast density and Ohio has been one of them since 2015.
“It's very important to make sure if they tell you have too dense breasts, and that you need some further other type of mammogram or ultrasound even or even an MRI to do it,” said Johnson.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Consulte Mais informação »
 Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Consulte Mais informação »
 Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Consulte Mais informação »
 Mammogram facilities must notify women if they have dense breast tissue, FDA new guidance sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Mammogram facilities must notify women if they have dense breast tissue, FDA new guidance sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Consulte Mais informação »
 Mammogram facilities must notify women if they have dense breast tissue, FDA new guidance sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Mammogram facilities must notify women if they have dense breast tissue, FDA new guidance sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Consulte Mais informação »
 Pfizer migraine nasal spray snags FDA approval for summer launchNews of the FDA’s approval came after two randomized, double-blind, placebo-controlled studies.
Pfizer migraine nasal spray snags FDA approval for summer launchNews of the FDA’s approval came after two randomized, double-blind, placebo-controlled studies.
Consulte Mais informação »