I am a London-based reporter for Forbes covering breaking news. Previously, I have worked as a reporter for a specialist legal publication covering big data and as a freelance journalist and policy analyst covering science, tech and health. I have a master’s degree in Biological Natural Sciences and a master’s degree in the History and Philosophy of Science from the University of Cambridge. Follow me on Twitter @theroberthart or email me at [email protected]
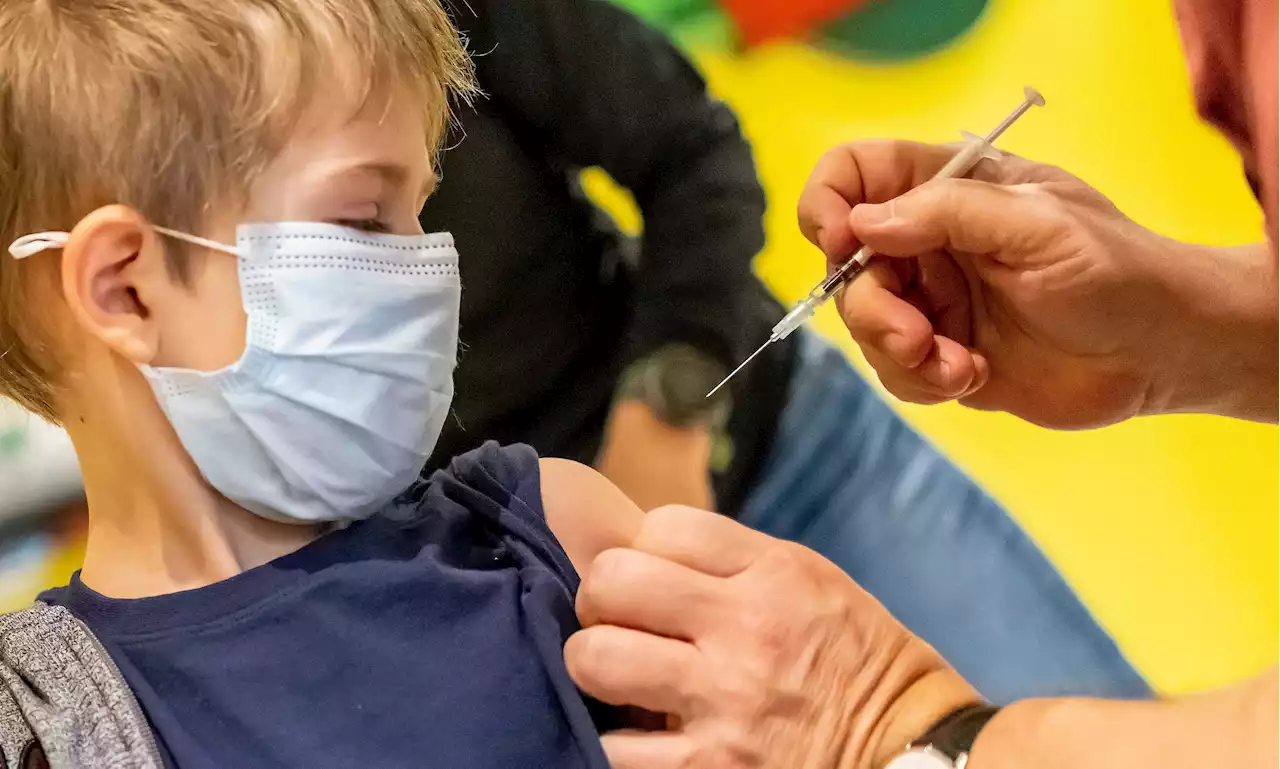
Moderna on Wednesday announced plans to seek emergency authorization for its Covid-19 vaccine in children under six after its clinical trial found two low doses were safe and generated a “robust” immune response, paving the way for the first shots for kids under five who are the last group of Americans still unable to get vaccinated against the coronavirus.Key Facts
The trial, which has not been peer reviewed, involved around 4,200 children aged between two and six years old and around 2,500 children between six months and two years old.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Moderna says its low-dose COVID shots work for kids under 6Moderna says its COVID-19 vaccine works in babies, toddlers and preschoolers
Moderna says its low-dose COVID shots work for kids under 6Moderna says its COVID-19 vaccine works in babies, toddlers and preschoolers
Consulte Mais informação »
 Moderna says its low-dose COVID shots work for kids under 6Moderna’s COVID-19 vaccine works in babies, toddlers and preschoolers the company announced Wednesday -- and if regulators agree it could mean a chance to finally start vaccinating the littlest kids by summer.
Moderna says its low-dose COVID shots work for kids under 6Moderna’s COVID-19 vaccine works in babies, toddlers and preschoolers the company announced Wednesday -- and if regulators agree it could mean a chance to finally start vaccinating the littlest kids by summer.
Consulte Mais informação »
 Moderna expands COVID-19 vaccines to treat related illnessesModerna said Tuesday that it would expand its COVID-19 vaccine to treat related illnesses.
Moderna expands COVID-19 vaccines to treat related illnessesModerna said Tuesday that it would expand its COVID-19 vaccine to treat related illnesses.
Consulte Mais informação »
 Vaccines Remain Effective against BA.2, but Protection from Infection Wanes over TimeSuch protection declines within months of the mRNA COVID vaccines’ third dose. Yet the vaccines continue to ward off severe disease
Vaccines Remain Effective against BA.2, but Protection from Infection Wanes over TimeSuch protection declines within months of the mRNA COVID vaccines’ third dose. Yet the vaccines continue to ward off severe disease
Consulte Mais informação »
Vaccines protect against infection from Omicron subvariant — but not for longTwo doses of COVID vaccine cut the risk of infection and mild illness from the rising BA.2 subvariant, although protection wanes quickly.
Consulte Mais informação »
 White House says administration is out of money for Covid testing, treatment and vaccines without new fundingThe White House said Monday that -- absent additional funding approval from Congress -- the administration is 'simply out' of funding for Covid-19 testing, treatment and vaccines.
White House says administration is out of money for Covid testing, treatment and vaccines without new fundingThe White House said Monday that -- absent additional funding approval from Congress -- the administration is 'simply out' of funding for Covid-19 testing, treatment and vaccines.
Consulte Mais informação »