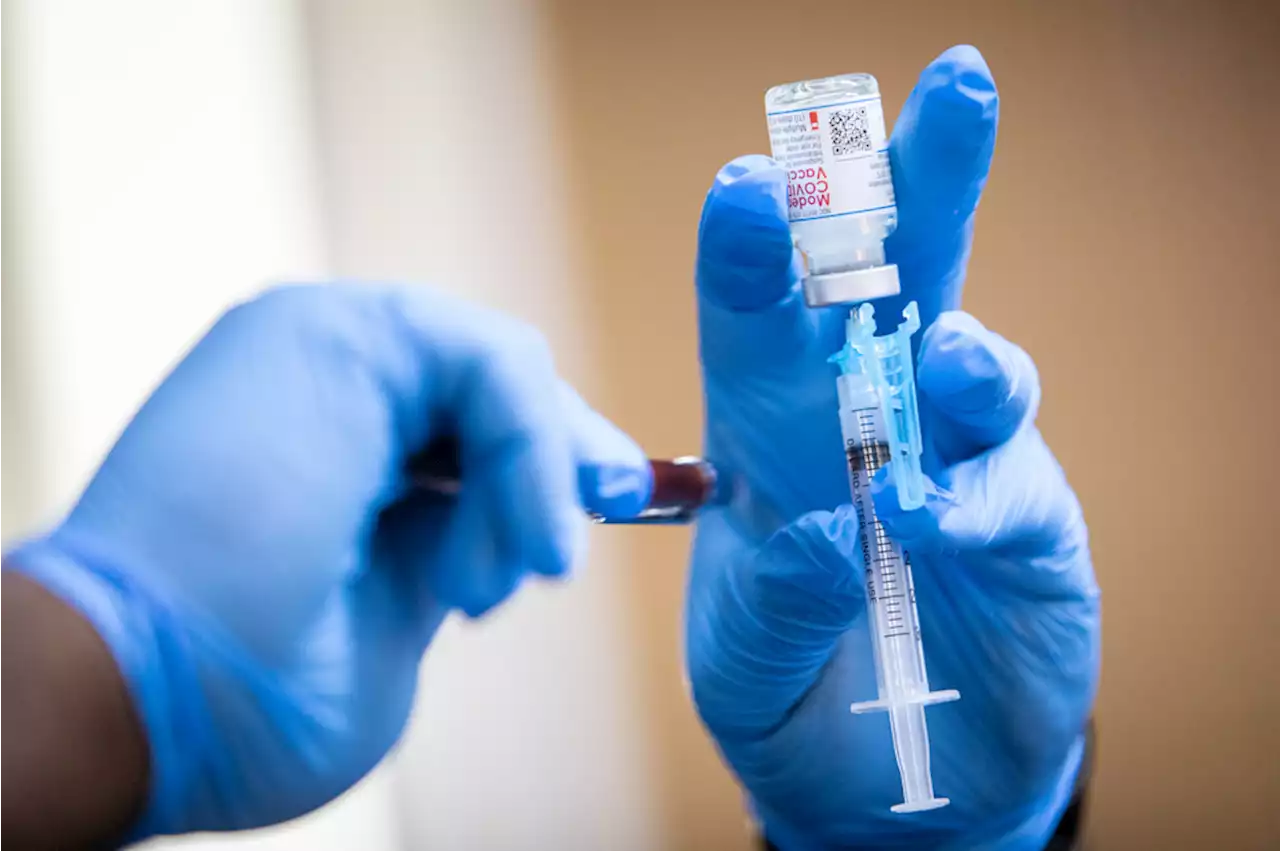
If approved, this would be the second booster shot Moderna has issued for people ages 18 and up.
"The request to include adults over 18 years of age was made to provide flexibility for the U.S. Centers for Disease Control and Prevention and healthcare providers to determine the appropriate use of an additional booster dose of mRNA-1273, including for those at higher risk of COVID-19..." the company said in a statement.
The decision to request an additional dose stemmed from data in the U.S. and Israel on the impacts of the Omicron variant, the statement said. The first dose, which was administered as a pair, was approved by the FDA under emergency use in December 2020, while Moderna's first booster shot
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
NPR Cookie Consent and Choices
Consulte Mais informação »
 UW Medicine seeks participants for Novavax booster studyUW Medicine seeks participants for a trial on Novavax's COVID-19 booster shot. FOX13
UW Medicine seeks participants for Novavax booster studyUW Medicine seeks participants for a trial on Novavax's COVID-19 booster shot. FOX13
Consulte Mais informação »
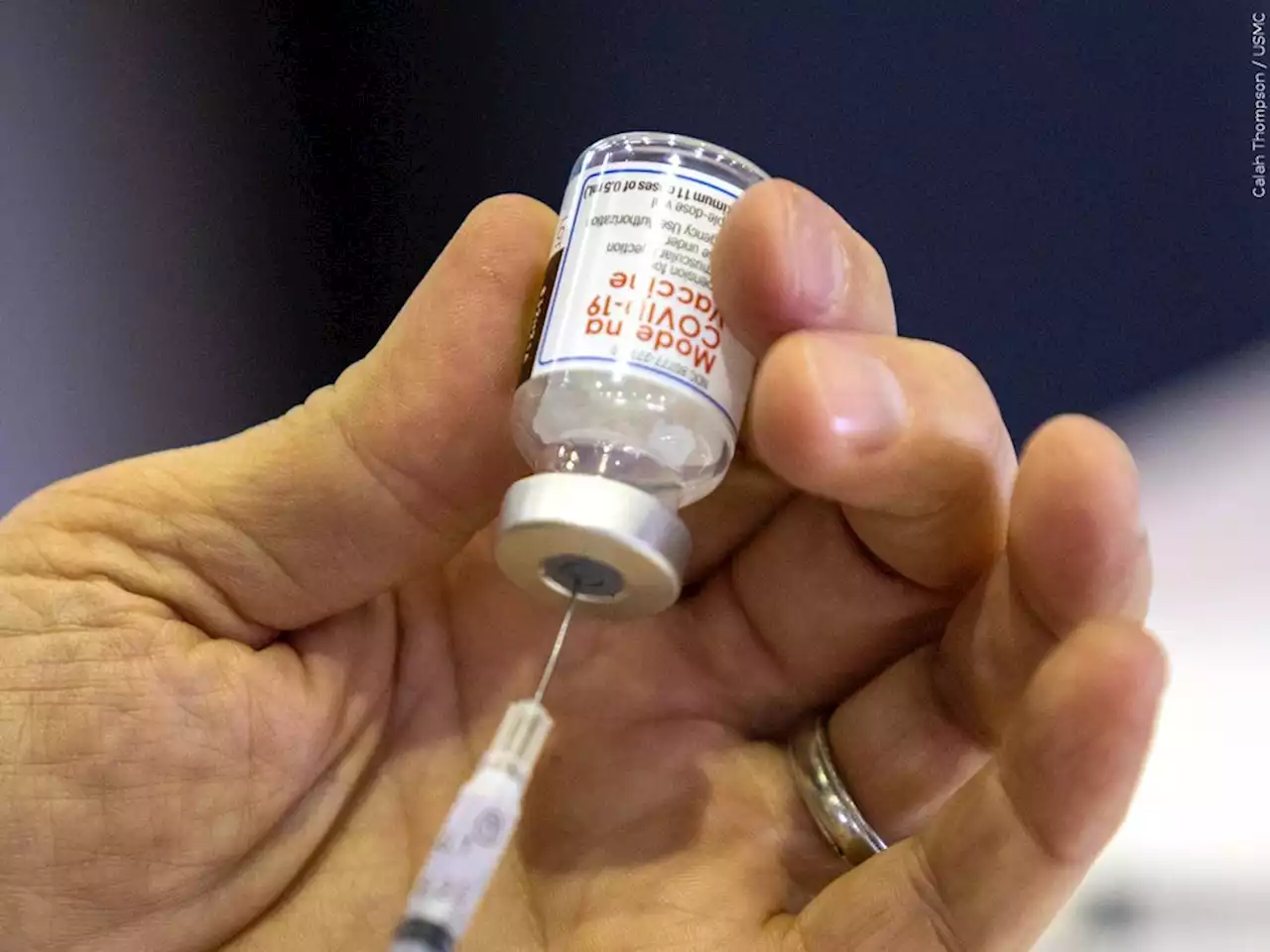 Moderna seeks FDA authorization for 4th dose of COVID shotThe request is broader than rival pharmaceutical company Pfizer’s request earlier this week for the regulator to approve a booster shot for all seniors.
Moderna seeks FDA authorization for 4th dose of COVID shotThe request is broader than rival pharmaceutical company Pfizer’s request earlier this week for the regulator to approve a booster shot for all seniors.
Consulte Mais informação »
 Moderna seeks FDA authorization for 4th dose of COVID shotDrugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna seeks FDA authorization for 4th dose of COVID shotDrugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Consulte Mais informação »
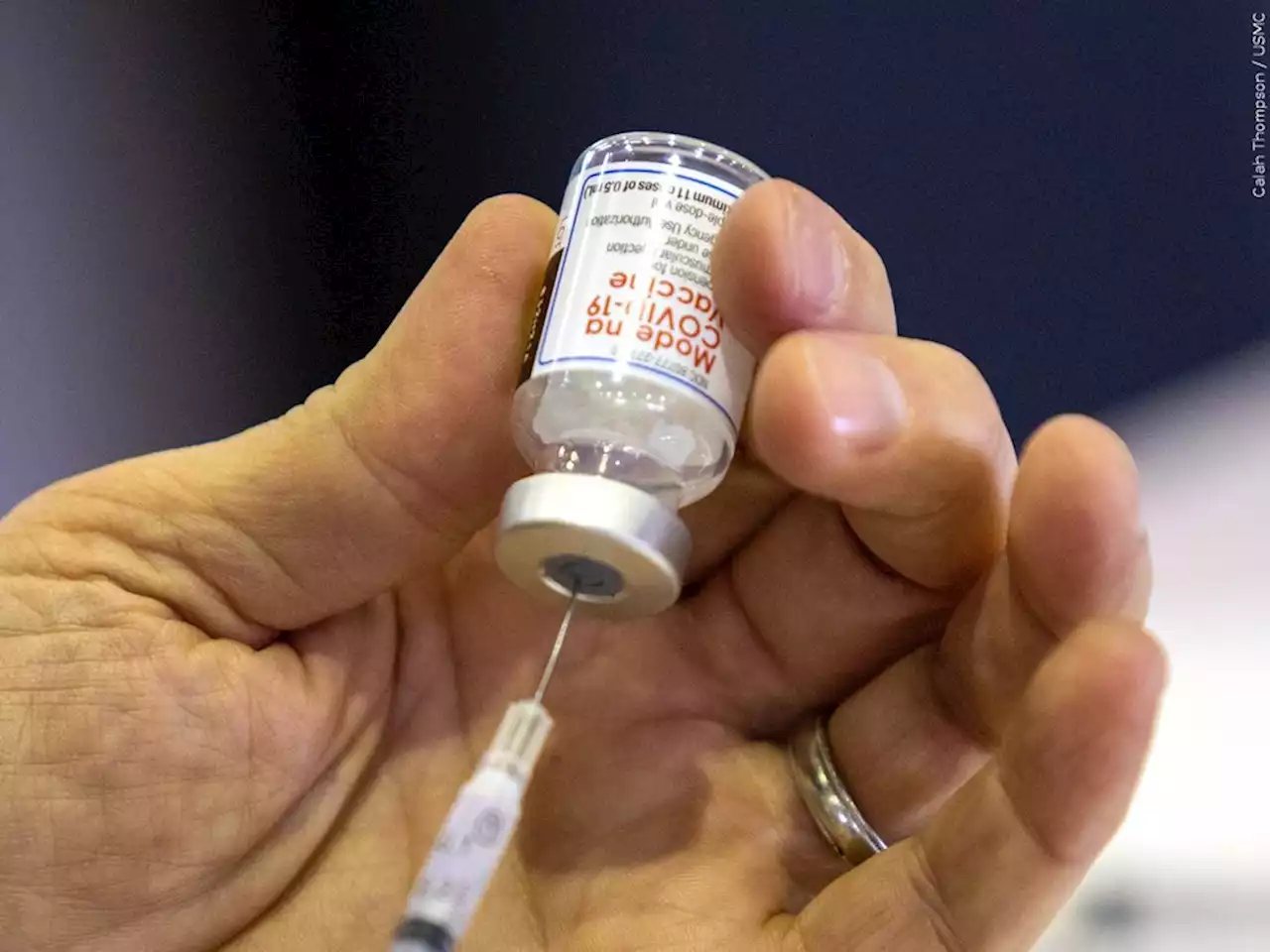 Moderna seeks FDA authorization for 4th dose of COVID shotDrugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Moderna seeks FDA authorization for 4th dose of COVID shotDrugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults.
Consulte Mais informação »
 Moderna seeks FDA authorization for 4th dose of COVID shotBREAKING: Drugmaker Moderna has asked the Food and Drug Administration to authorize the fourth shot of its COVID-19 vaccine as a booster dose for all adults
Moderna seeks FDA authorization for 4th dose of COVID shotBREAKING: Drugmaker Moderna has asked the Food and Drug Administration to authorize the fourth shot of its COVID-19 vaccine as a booster dose for all adults
Consulte Mais informação »