A consumer advocacy group has asked the FDA to remove it from foods and drinks. But the dye industry defends its safety.
The International Association of Color Manufacturers referencedthat found no ill effects on thyroid function among 30 men, divided into three groups with each taking a different daily dose of red dye No. 3, including 20, 60 and 200 milligrams. The study — because it only lasted two weeks — did not address long-term exposure and the risk of cancer.
Regarding the cancer study in rats, however, Sarah Codrea, the organization’s executive director, said that “effects in experimental animals were observed at doses at least 60-fold higher than the levels of no effect observed in the human study.”Animal research typically involves administering high doses in small numbers of animals to compensate for their shorter life spans, in this case, about two years for rodents.
Brian Ronholm, director of food policy for Consumer Reports — which also signed on to the petition — agreed. “It’s frustrating and completely illogical,” he said. In 1960, the FDA gave “provisional” — that is, temporary — permission to add the dye to both cosmetics and foods. In 1969, before studies suggested it was carcinogenic, the FDA allowed for its “permanent” use in foods, but kept its provisional status in cosmetics, pending the outcome of skin exposure studies.
After making the cancer connection in animals, the agency banned its use in cosmetics, where it had been allowed temporarily, and laterto remove it from food. But it never did because a permanent designation is tantamount to approval; and once approved, it’s hard to get rid of it. “It’s easier to deny a petition to permanently list an additive — as the FDA did with cosmetics — than it is to revoke an approval,” Lefferts said.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Proposed eased blood donor restrictions has South Texas Blood and Tissue Center hopefulThe blood donor pool could soon grow as the FDA is considering easing restrictions for the LGBTQIA+ community.
Proposed eased blood donor restrictions has South Texas Blood and Tissue Center hopefulThe blood donor pool could soon grow as the FDA is considering easing restrictions for the LGBTQIA+ community.
Consulte Mais informação »
 FDA Approves Abrocitinib for Adolescents With Atopic DermatitisFDA expanded the indication for Pfizer's oral JAK inhibitor for atopicdermatitis, abrocitinib, to include adolescents aged 12 through 17 years. DermTwitter
FDA Approves Abrocitinib for Adolescents With Atopic DermatitisFDA expanded the indication for Pfizer's oral JAK inhibitor for atopicdermatitis, abrocitinib, to include adolescents aged 12 through 17 years. DermTwitter
Consulte Mais informação »
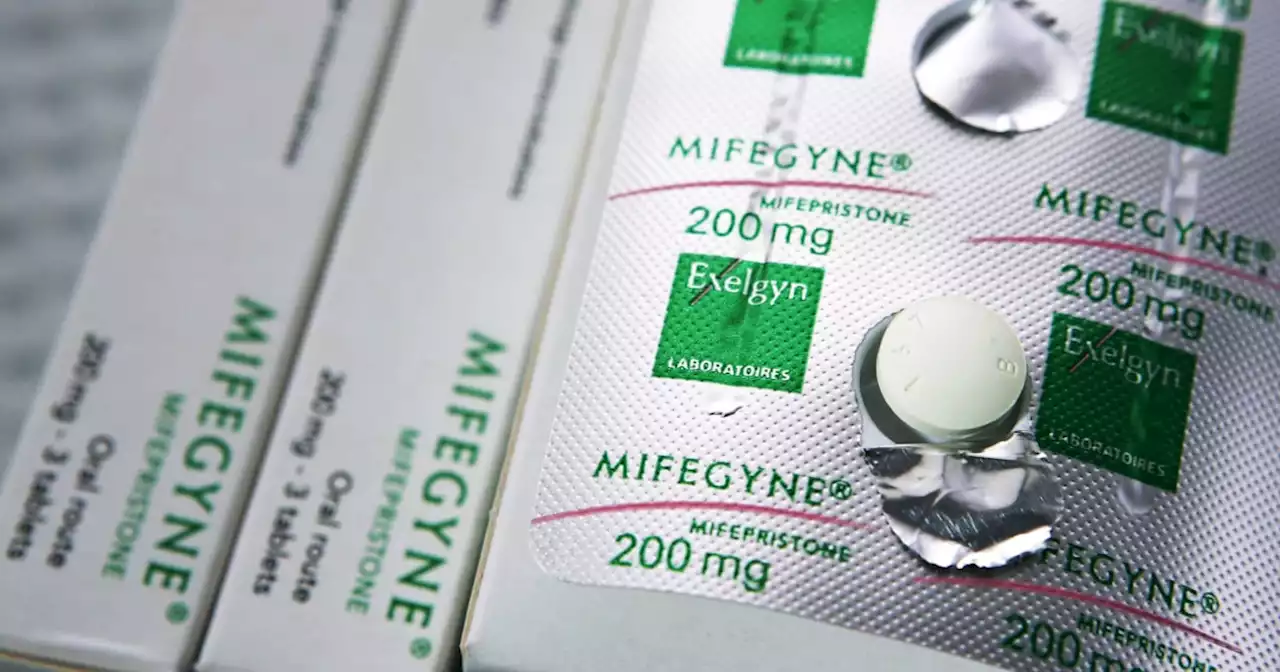 In lawsuit challenging FDA approval of abortion pills, state attorneys general weigh inA decision from a Trump-appointed judge could come later this month in a lawsuit alleging that the FDA’s approval of two abortion medications was unlawful.
In lawsuit challenging FDA approval of abortion pills, state attorneys general weigh inA decision from a Trump-appointed judge could come later this month in a lawsuit alleging that the FDA’s approval of two abortion medications was unlawful.
Consulte Mais informação »
 40 million would lose abortion access if court blocks pill, study shows40 million more women would lose access to abortion care if a federal court revokes the use of a key drug in medication abortions, data shows.
40 million would lose abortion access if court blocks pill, study shows40 million more women would lose access to abortion care if a federal court revokes the use of a key drug in medication abortions, data shows.
Consulte Mais informação »
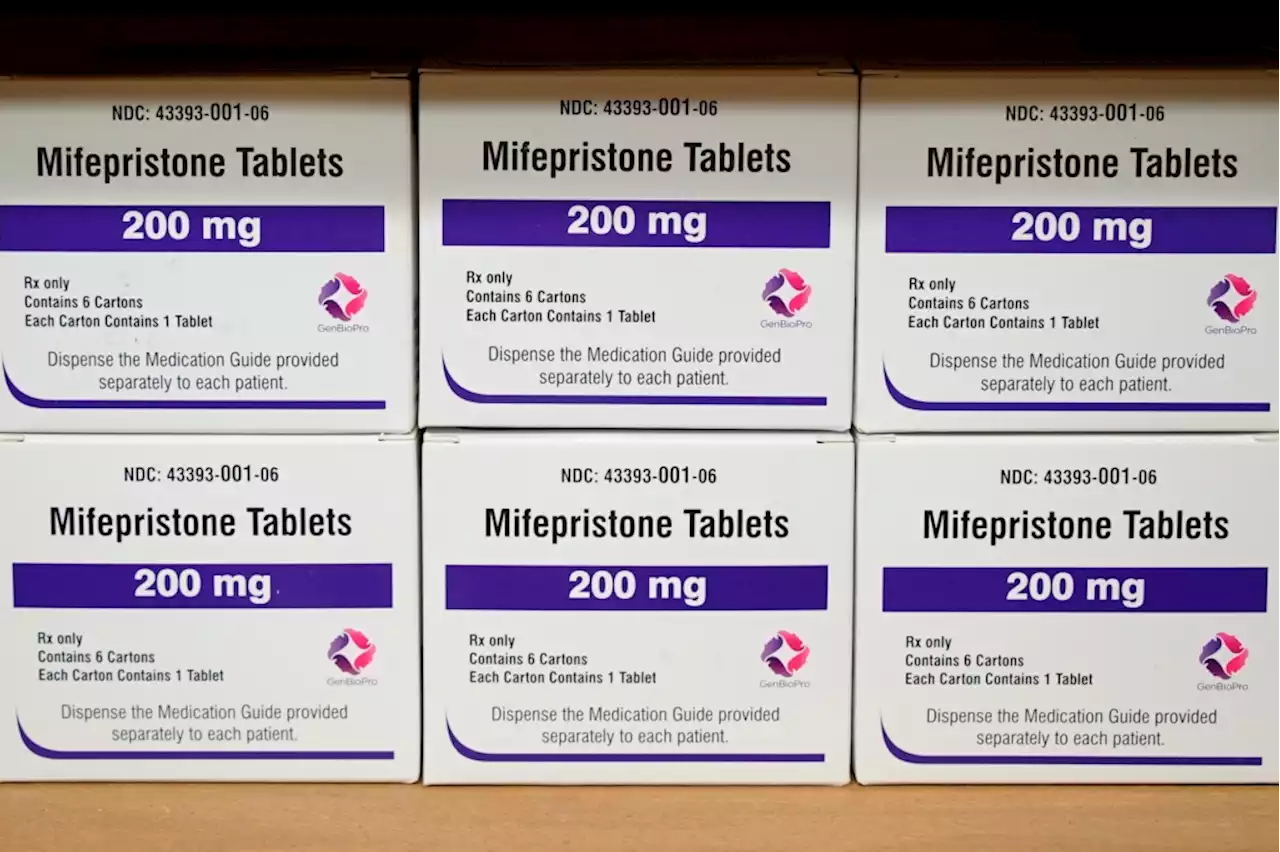 Texas ruling could pull abortion pill from whole countryHalting access to the drug more than 20 years after FDA approval would be “extraordinary and unprecedented,” federal attorneys stated in a legal filing.
Texas ruling could pull abortion pill from whole countryHalting access to the drug more than 20 years after FDA approval would be “extraordinary and unprecedented,” federal attorneys stated in a legal filing.
Consulte Mais informação »
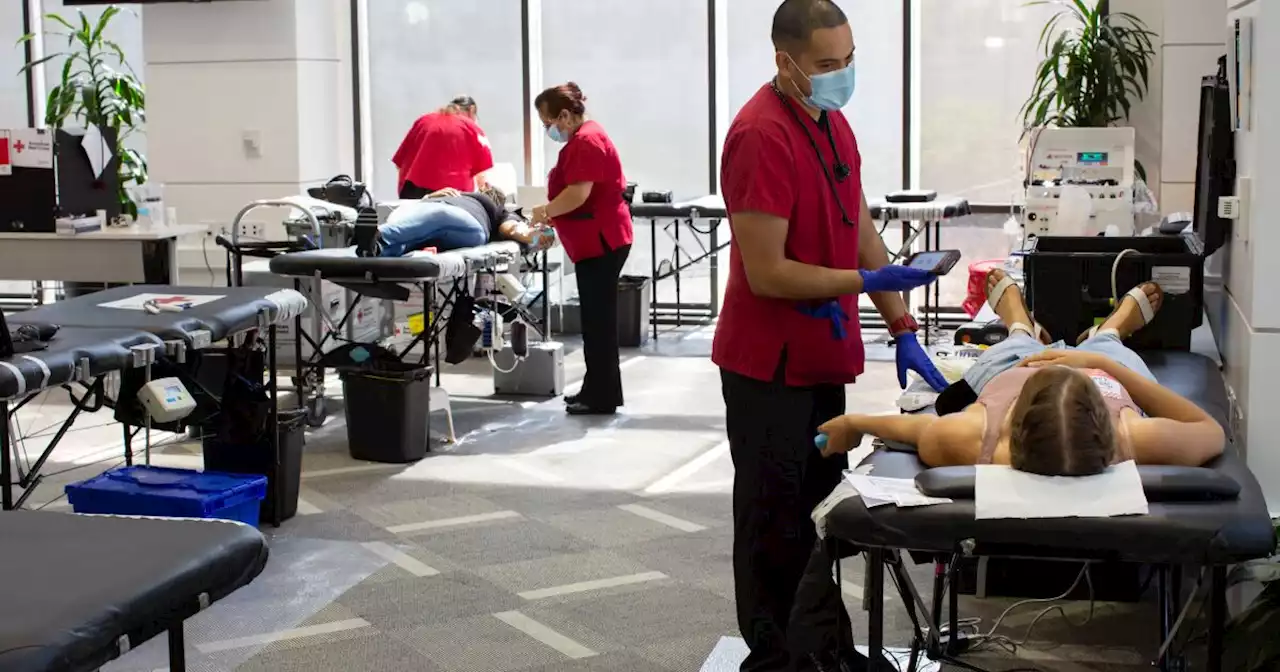 Opinion: Will new blood donation rules finally stop stigmatizing gay men?The FDA's revised guidelines would loosen bans on some gay and bisexual men donating blood, but keep other dubious restrictions.
Opinion: Will new blood donation rules finally stop stigmatizing gay men?The FDA's revised guidelines would loosen bans on some gay and bisexual men donating blood, but keep other dubious restrictions.
Consulte Mais informação »
