Eisai and Biogen share clinical trial data confirming that lecanemab slows mental decline amid reports of potentially related deaths
Researchers have got a first look at phase III clinical trial data for a much lauded experimental Alzheimer’s drug—and although the data support it having a moderate cognitive benefit for people, scientists worry about its safety.
“It’s quite a complicated balancing act for risks and benefits,” says Rob Howard, a psychiatrist at University College London who specializes in dementia. And he worries about how patients and families who are desperate for Alzheimer’s treatments will weigh the two sides, if lecanemab is approved by regulatory agencies.
Benefits and risks Researchers are glad to see the swift publication of the lecanemab trial data. Some have previously criticized the rollout of another monoclonal antibody treatment for Alzheimer’s: aducanumab. Like lecanemab, aducanumab was designed to sweep clumps of a protein called amyloid-β from the brain; many researchers think this protein is a root cause of Alzheimer’s.
But some researchers have questioned whether this shift is big enough to be noticeable in a person. A one-point difference on the CDR-SB is the minimum to be clinically important, Howard says. During Clarity AD, 13 people taking lecanemab developed symptomatic brain bleeds—or strokes—whereas only 2 people in the placebo group did, according to the conference presentation. This represents just 1.4% of the treatment group, Howard says, but “that’s not a trivial risk profile”.
Because of the tie with anticoagulants and other factors, it’s a bit difficult to detangle whether lecanemab played a role in the deaths, said Marwan Sabbagh, a neurologist at the Barrow Neurological Institute in Phoenix, Arizona, while presenting data at the conference. “These things are continuing to be explored,” he said. Although the rate of brain hemorrhage is low with lecanemab, it does rise with anticoagulants, he added.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Alzheimer's drug from Eisai and Biogen slows cognitive decline, side-effects in focusThe 18-month trial enrolled nearly 1,800 participants.
Alzheimer's drug from Eisai and Biogen slows cognitive decline, side-effects in focusThe 18-month trial enrolled nearly 1,800 participants.
Consulte Mais informação »
 Heralded Alzheimer’s drug works — but safety concerns loomEisai and Biogen share clinical trial data confirming that lecanemab slows mental decline amid reports of potentially related deaths.
Heralded Alzheimer’s drug works — but safety concerns loomEisai and Biogen share clinical trial data confirming that lecanemab slows mental decline amid reports of potentially related deaths.
Consulte Mais informação »
 New Alzheimer’s drug shows promise in slowing disease but may contain significant risks, study showsAn experimental Alzheimer’s drug modestly slowed the brain disease’s inevitable worsening, researchers report — but it remains unclear how much difference that might make in people’s lives.
New Alzheimer’s drug shows promise in slowing disease but may contain significant risks, study showsAn experimental Alzheimer’s drug modestly slowed the brain disease’s inevitable worsening, researchers report — but it remains unclear how much difference that might make in people’s lives.
Consulte Mais informação »
 Drug slows Alzheimer’s but can it make a real difference?Japanese drugmaker Eisai and its U.S. partner Biogen announced earlier this fall that lecanemab appears to work, a badly needed bright spot after disappointments in the quest for better Alzheimer's treatments.
Drug slows Alzheimer’s but can it make a real difference?Japanese drugmaker Eisai and its U.S. partner Biogen announced earlier this fall that lecanemab appears to work, a badly needed bright spot after disappointments in the quest for better Alzheimer's treatments.
Consulte Mais informação »
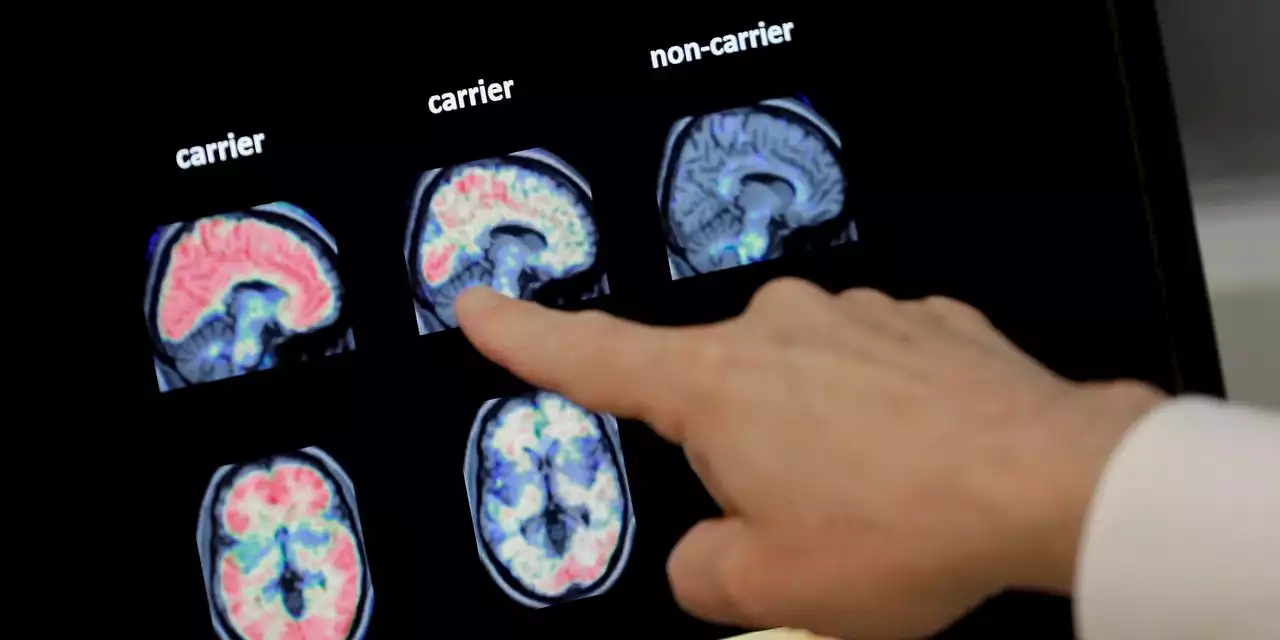 Newest Alzheimer’s Drugs Take Large but Fraught Step Toward ApprovalPositive study results for Eisai-Biogen lifted the prospects for its medication and one from Eli Lilly—although regulators will have to weigh risks.
Newest Alzheimer’s Drugs Take Large but Fraught Step Toward ApprovalPositive study results for Eisai-Biogen lifted the prospects for its medication and one from Eli Lilly—although regulators will have to weigh risks.
Consulte Mais informação »
 Drug slows Alzheimer’s but can it make a real difference?Japanese drugmaker Eisai and its U.S. partner Biogen announced earlier this fall that lecanemab appears to work, a badly needed bright spot after disappointments in the quest for better Alzheimer's treatments.
Drug slows Alzheimer’s but can it make a real difference?Japanese drugmaker Eisai and its U.S. partner Biogen announced earlier this fall that lecanemab appears to work, a badly needed bright spot after disappointments in the quest for better Alzheimer's treatments.
Consulte Mais informação »