Pfizer’s vaccine candidate, which is pending FDA review, is administered to pregnant women, who then pass the protective antibodies to the baby.
RSV is a common respiratory virus that usually causes mild, cold-like symptoms.that can cause severe breathing problems, could be approved by August. that the U.S. Food and Drug Administration had accepted its application for review and has set an action date of deciding whether to approve or not by August 2023.
"We look forward to progressing the review of Pfizer’s RSV maternal vaccine candidate with the FDA and other regulatory authorities, given its significant potential to positively contribute to global health in the prevention of RSV in infants," Anderson added. While everyone can get RSV, it causes the most threat to infants, older adults, and other vulnerable people, who can get serious airway and lung infections, according to the
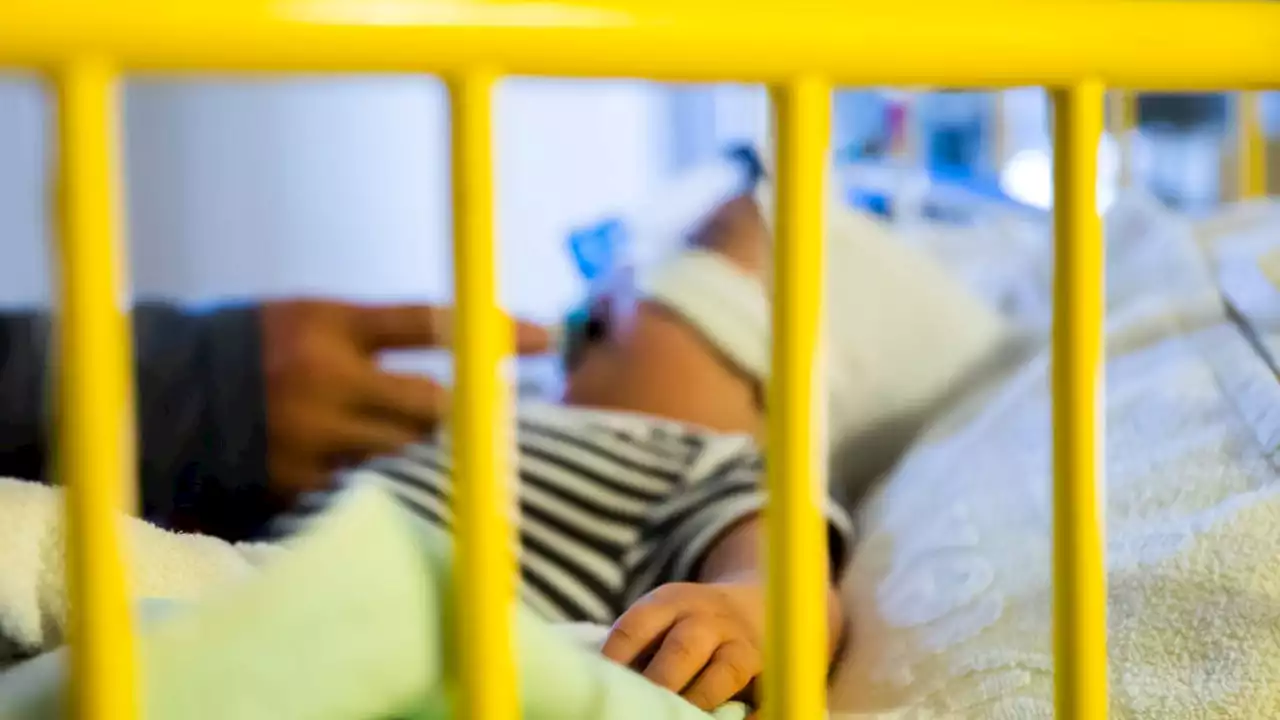
FILE - A father cares for his 8-and-a-half-month-old son, who is in the intensive care unit of the pediatric clinic at a hospital with a respiratory infection.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 1st RSV vaccine could be approved by August, pending FDA reviewThe FDA will decide whether or not to approve an RSV vaccine designed to protect newborns by August 2023.
1st RSV vaccine could be approved by August, pending FDA reviewThe FDA will decide whether or not to approve an RSV vaccine designed to protect newborns by August 2023.
Consulte Mais informação »
 1st RSV vaccine could be approved by August, pending FDA reviewPfizer's vaccine would be the first RSV vaccine given to pregnant people to protect infants. The company said that there were 'no safety concerns' for vaccinated pregnant participants and their newborns during the trial.
1st RSV vaccine could be approved by August, pending FDA reviewPfizer's vaccine would be the first RSV vaccine given to pregnant people to protect infants. The company said that there were 'no safety concerns' for vaccinated pregnant participants and their newborns during the trial.
Consulte Mais informação »
 Pfizer RSV Vaccine That Protects Infants Could Receive FDA Approval This SummerThe single-dose RSV vaccine developed by Pfizer is administered to expectant mothers in the late second-to-third trimester of their pregnancy.
Pfizer RSV Vaccine That Protects Infants Could Receive FDA Approval This SummerThe single-dose RSV vaccine developed by Pfizer is administered to expectant mothers in the late second-to-third trimester of their pregnancy.
Consulte Mais informação »
 1st RSV vaccine could be approved by August, pending FDA reviewPfizer's vaccine would be the first RSV vaccine given to pregnant people to protect infants. The company said that there were 'no safety concerns' for vaccinated pregnant participants and their newborns during the trial.
1st RSV vaccine could be approved by August, pending FDA reviewPfizer's vaccine would be the first RSV vaccine given to pregnant people to protect infants. The company said that there were 'no safety concerns' for vaccinated pregnant participants and their newborns during the trial.
Consulte Mais informação »
 FDA agrees to review Pfizer's RSV vaccine for pregnant individualsThe Food and Drug Administration has accepted an application from Pfizer to review its RSV vaccine for pregnant individuals.
FDA agrees to review Pfizer's RSV vaccine for pregnant individualsThe Food and Drug Administration has accepted an application from Pfizer to review its RSV vaccine for pregnant individuals.
Consulte Mais informação »
 RSV vaccines to receive review by FDA advisory panelA vaccine advisory committee for the Food and Drug Administration will review whether to recommend two vaccines for respiratory syncytial virus for approval next week as pharmaceutical giant Pfizer and rival drug manufacturer GSK vie to have the first RSV vaccine to hit the U.S. market.
RSV vaccines to receive review by FDA advisory panelA vaccine advisory committee for the Food and Drug Administration will review whether to recommend two vaccines for respiratory syncytial virus for approval next week as pharmaceutical giant Pfizer and rival drug manufacturer GSK vie to have the first RSV vaccine to hit the U.S. market.
Consulte Mais informação »