Parenting expert Dr. Sheryl Ziegler discusses the warning signs of relational aggression during Bullying Awareness Month.
against using 26 different types of over-the-counter eye drops because they aren't sterile, increasing the risk for infections that can potentially blind users. According to the U.S. Food and Drug Administration, the products are supposed to be sterile to prevent eye infections. Eye drops pose a heightened risk of passing along harmful infections because drugs applied directly to the eyes bypass some of the body's natural defenses.
CVS, Rite Aid and Target have all agreed to remove the affected products from store shelves, although Leader, Rugby and Velocity eye drops are still available online and in some retail stores, the FDA noted. The FDA said it recommended Wednesday that the manufacturer recall all of the 26 varieties of eye drops. The warning was issued after investigators found"insanitary conditions" and bacterial contamination in critical parts of the manufacturing plant where the eye drops are made.
The FDA says they have not received any report of infections or other adverse effects related to the affected eye drops, but urged anybody with symptoms of an eye infection after using one to seek medical aid immediately. FDA warns to stop using 2 eye drop productsKUSA would like to send you push notifications about the latest news and weather.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 The FDA warns consumers to stop using several eyedrop products due to infection riskFederal health officials issued the alert for six different brands of products after finding bacterial contamination at a manufacturing facility.
The FDA warns consumers to stop using several eyedrop products due to infection riskFederal health officials issued the alert for six different brands of products after finding bacterial contamination at a manufacturing facility.
Consulte Mais informação »
 Recall alert: FDA warns against using 26 over-the-counter eye dropsThe Food and Drug Administration has announced the recall of 26 different types of eye drops, all of which were sold over-the-counter.
Recall alert: FDA warns against using 26 over-the-counter eye dropsThe Food and Drug Administration has announced the recall of 26 different types of eye drops, all of which were sold over-the-counter.
Consulte Mais informação »
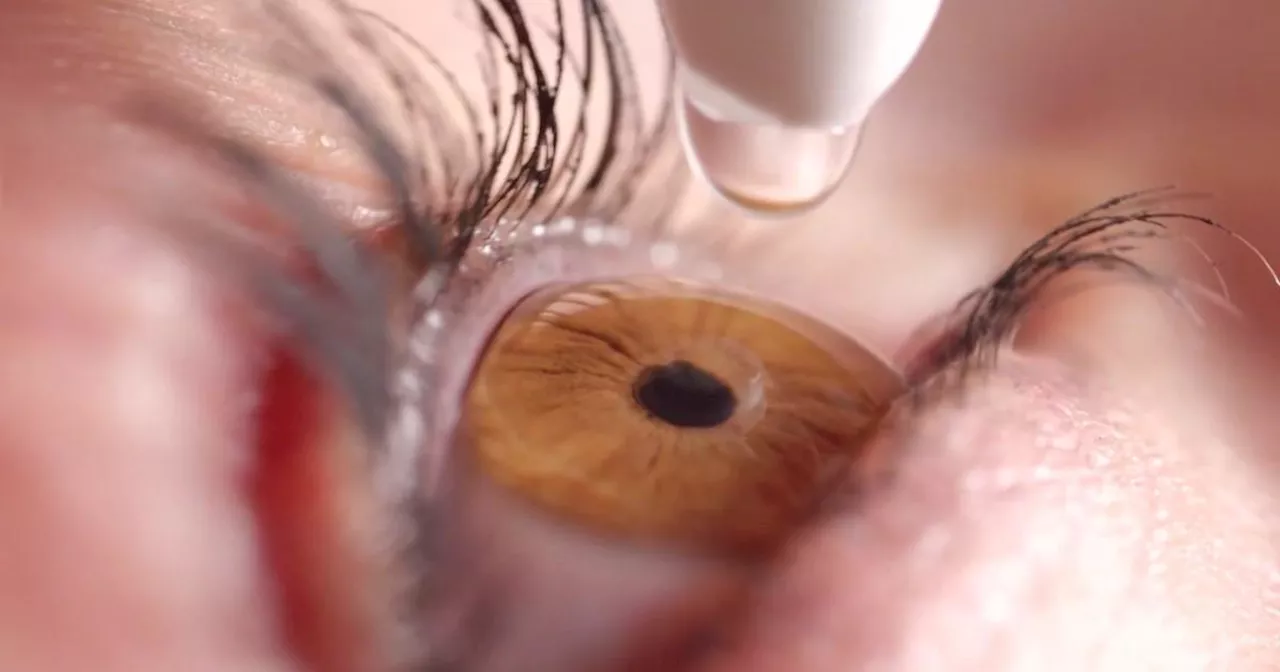 FDA warns consumers against using 26 eye drop products because of infection riskRegulators are urging people to dispose of eye drop products sold under brands such as CVS Health, Rite Aid and Target Up & Up.
FDA warns consumers against using 26 eye drop products because of infection riskRegulators are urging people to dispose of eye drop products sold under brands such as CVS Health, Rite Aid and Target Up & Up.
Consulte Mais informação »
 FDA warns against using 26 eye drop products over infection riskThe FDA said use of the drops has a 'potential risk of eye infections that could result in partial vision loss or blindness.'
FDA warns against using 26 eye drop products over infection riskThe FDA said use of the drops has a 'potential risk of eye infections that could result in partial vision loss or blindness.'
Consulte Mais informação »
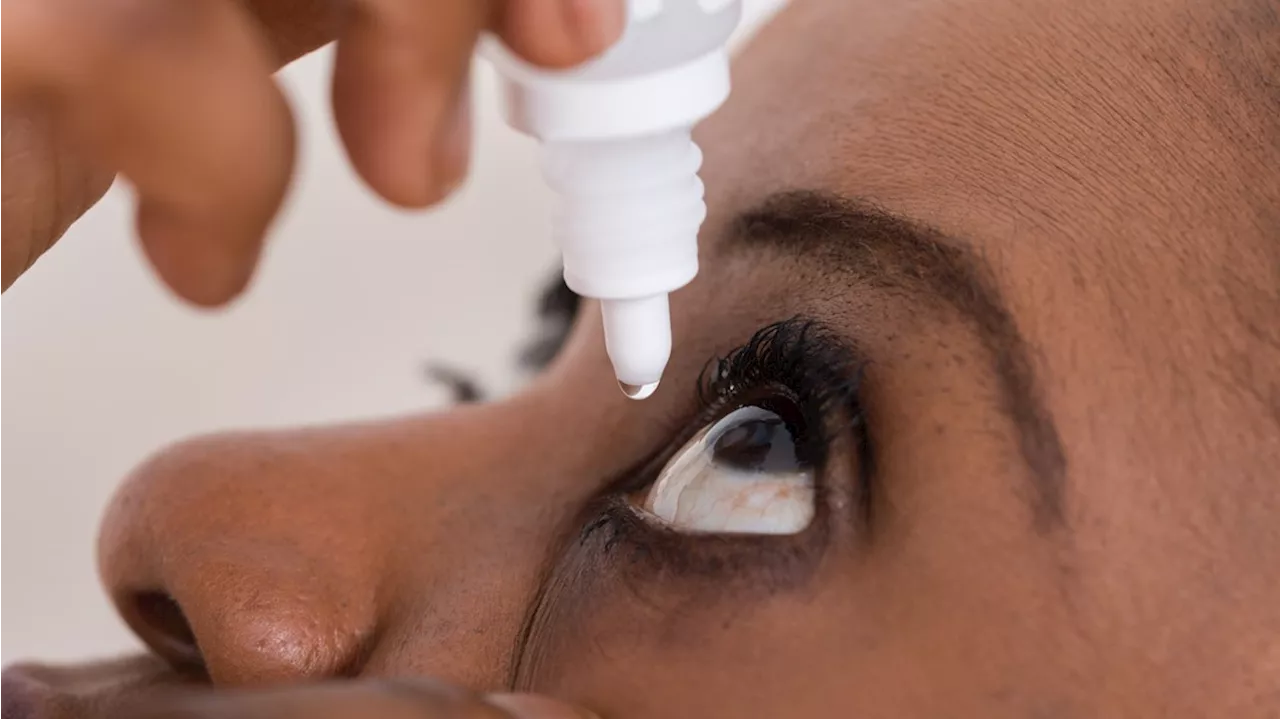 FDA warns to stop using 26 eye drop productsTexas Attorney General Ken Paxton will be back in a courtroom today but not to represent the state. He will defend himself against accusations of fraud.
FDA warns to stop using 26 eye drop productsTexas Attorney General Ken Paxton will be back in a courtroom today but not to represent the state. He will defend himself against accusations of fraud.
Consulte Mais informação »
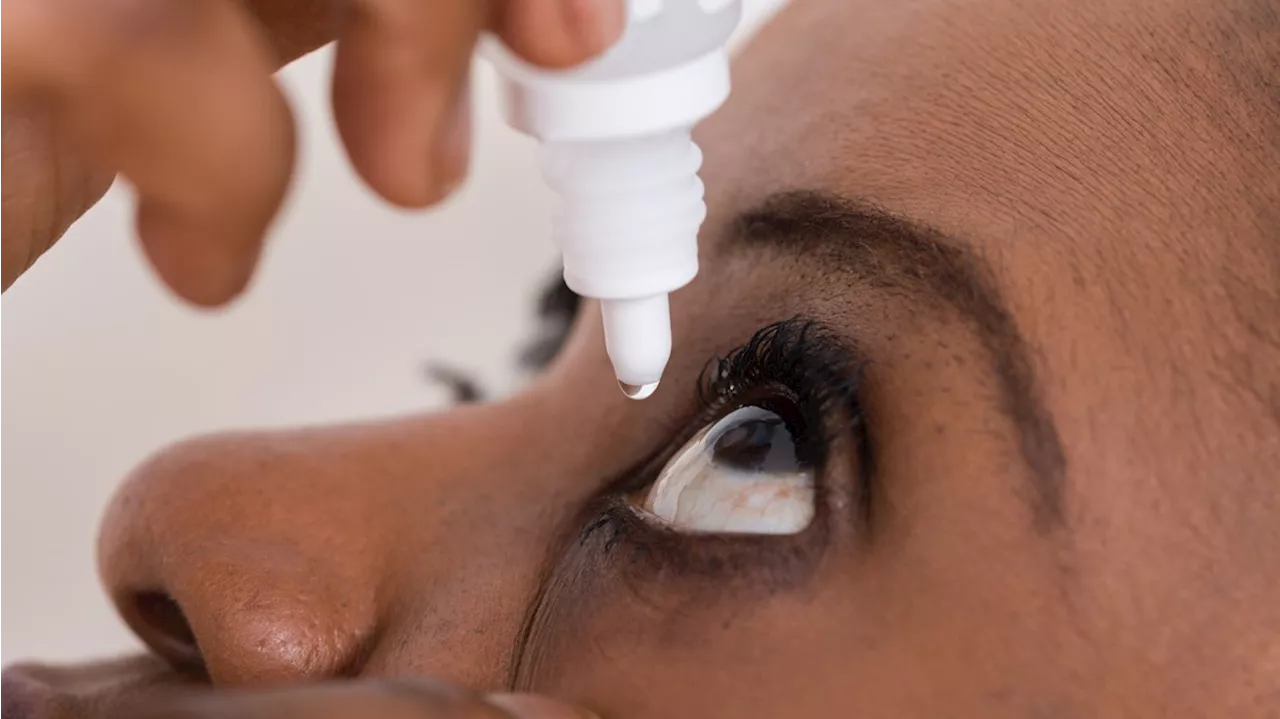 FDA warns to stop using 26 eye drop productsThe state is requesting $150,000 to study the effectiveness of distracted driving zones in our state.
FDA warns to stop using 26 eye drop productsThe state is requesting $150,000 to study the effectiveness of distracted driving zones in our state.
Consulte Mais informação »
