The FDA stopped short of saying the potentially life-threatening condition was caused by the drugs, which have become popular for weight loss.
However, the FDA stopped short of directly blaming the potentially life-threatening condition on the drug.
"Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure," the label reads. The FDA has received 8,571 reports of gastrointestinal disorders after use of semaglutide medications, which includes both Ozempic and Wegovy, according toIleus is specifically mentioned as a reaction in 33 cases listed on the FDA's dashboard of people taking semaglutide, including two deaths.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 What’s the Difference Between Mounjaro Vs. Ozempic? Experts ExplainDoctors break it down.
What’s the Difference Between Mounjaro Vs. Ozempic? Experts ExplainDoctors break it down.
Consulte Mais informação »
 Prescriptions for Ozempic and similar drugs soar past 9 millionA new analysis found that prescriptions for Ozempic and similar drugs quadrupled in less than three years, with many patients using the diabetes drug to lose weight.
Prescriptions for Ozempic and similar drugs soar past 9 millionA new analysis found that prescriptions for Ozempic and similar drugs quadrupled in less than three years, with many patients using the diabetes drug to lose weight.
Consulte Mais informação »
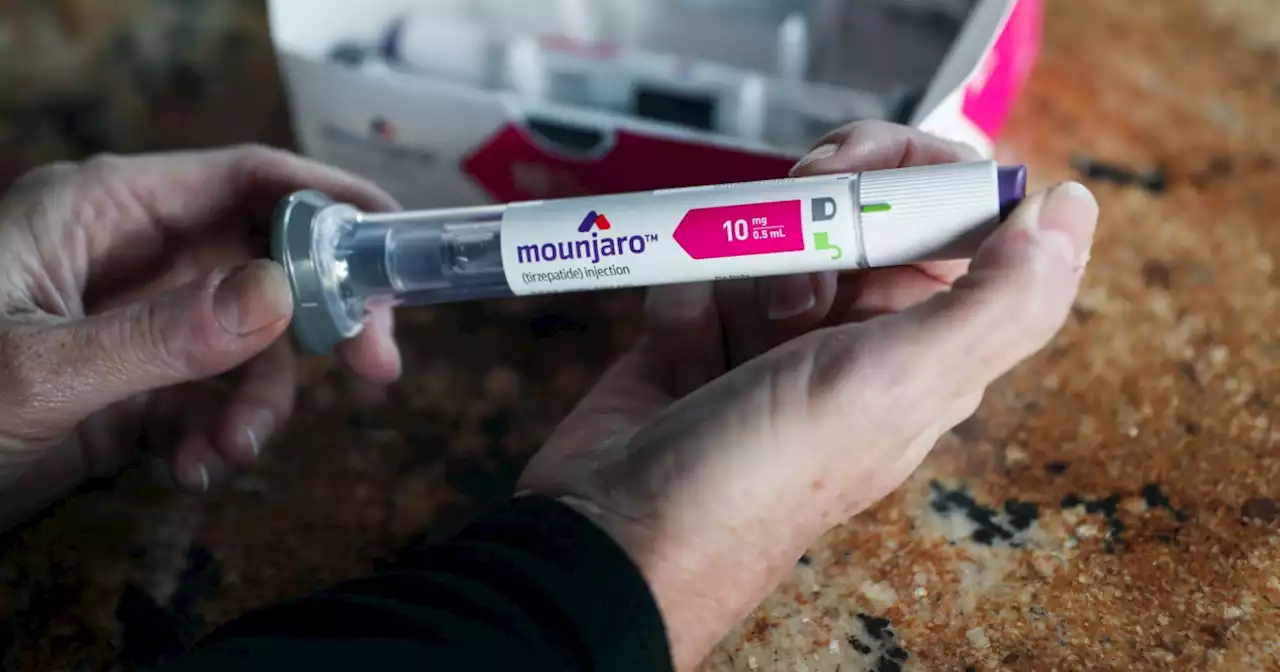 Watch out, Ozempic? Another diabetes drug is 'superior' for weight loss in studiesType 2 diabetes treatment tirzepatide, which is sold under the brand name Mounjaro, awaits FDA approval as a weight-loss medication.
Watch out, Ozempic? Another diabetes drug is 'superior' for weight loss in studiesType 2 diabetes treatment tirzepatide, which is sold under the brand name Mounjaro, awaits FDA approval as a weight-loss medication.
Consulte Mais informação »
 | FDA advisers weigh divisive ALS drug todayThere's a heated debate over the investigational treatment called NurOwn.
| FDA advisers weigh divisive ALS drug todayThere's a heated debate over the investigational treatment called NurOwn.
Consulte Mais informação »
 Viatris and Ocuphire Pharma get FDA green light for eye treatmentViatris Inc. and Ocuphire Pharma Inc. said Wednesday that the U.S. Food and Drug Administration has approved Ryzumvi to treat the side effects of...
Viatris and Ocuphire Pharma get FDA green light for eye treatmentViatris Inc. and Ocuphire Pharma Inc. said Wednesday that the U.S. Food and Drug Administration has approved Ryzumvi to treat the side effects of...
Consulte Mais informação »
FDA to Review Experimental ALS Treatment This WeekThe Food and Drug Administration meets this week to consider approval of an experimental treatment for Lou Gehrig's disease, the culmination of a yearslong lobbying effort by patients with the fatal neurodegenerative disease. Those advocates still face one giant hurdle: FDA...
Consulte Mais informação »
