The FDA announced its initial strategy to boost and strengthen the management of the country's supply of infant formula.
came just ahead of a hearing of the House Oversight and Accountability Committee about what went wrong during last year's infant formula shortage.
"The nation remains one outbreak, one tornado, flood or cyberattack away from finding itself in a similar place to that of February 17, 2022." "Clearly, I really wish, and I should have been notified sooner, so I could have initiated containment steps earlier. Had that happened, I believe we might not be here today," Yiannas said Tuesday. "Had the agency responded quicker to some of the earlier signals, I believe this crisis could have been averted or at least the magnitude lessened."
The agency said it will work with the industry on redundancy risk management plans that will help companies identify possible supply chain problems. It will also continue to enhance inspections of infant formula plants by expanding and improving training for agency investigators. The new strategy is just a first step; the long-term strategy is expected to be released in early 2024.
One positive development that came out of the crisis is that manufacturers are reporting formula volume to the FDA on a weekly basis even though there is no legal requirement to do so, he said.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA approves over-the-counter Narcan to reduce drug overdosesBREAKING: FDA approves the overdose reversal drug Narcan for over-the-counter use amid the ongoing opioid epidemic.
FDA approves over-the-counter Narcan to reduce drug overdosesBREAKING: FDA approves the overdose reversal drug Narcan for over-the-counter use amid the ongoing opioid epidemic.
Consulte Mais informação »
 FDA Approves First Over-the-Counter Drug That Reverses Opioid OverdosesNEW: The FDA approves selling Narcan without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter.
FDA Approves First Over-the-Counter Drug That Reverses Opioid OverdosesNEW: The FDA approves selling Narcan without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter.
Consulte Mais informação »
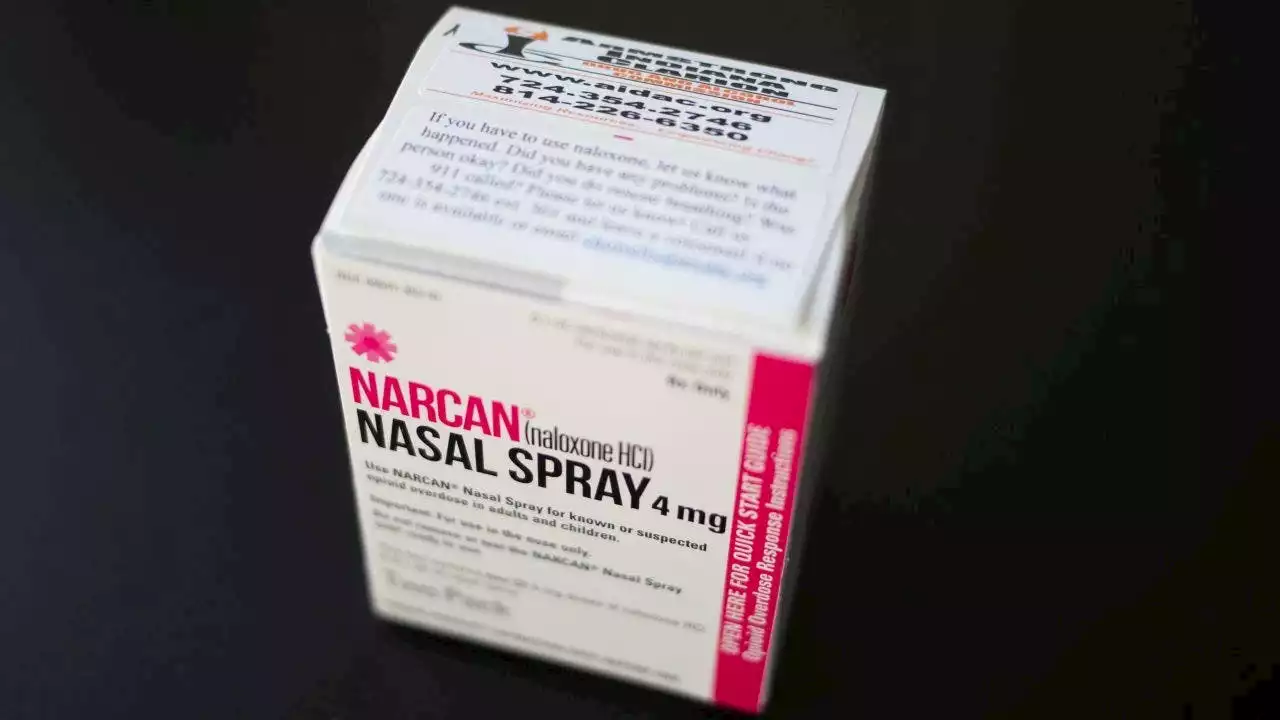 Opioid overdose drug Narcan approved for over-the-counter use by FDAThe U.S. Food and Drug Administration announced Wednesday it approved selling the life-saving naloxone-based nasal spray Narcan without a prescription.
Opioid overdose drug Narcan approved for over-the-counter use by FDAThe U.S. Food and Drug Administration announced Wednesday it approved selling the life-saving naloxone-based nasal spray Narcan without a prescription.
Consulte Mais informação »
 US FDA approves first OTC opioid overdose reversal drugThe U.S. Food and Drug Administration on Wednesday approved using Emergent BioSolutions Inc's Narcan without a prescription, paving the way for easy availability of the life-saving medication that is used to reverse opioid overdose.
US FDA approves first OTC opioid overdose reversal drugThe U.S. Food and Drug Administration on Wednesday approved using Emergent BioSolutions Inc's Narcan without a prescription, paving the way for easy availability of the life-saving medication that is used to reverse opioid overdose.
Consulte Mais informação »
 The FDA has approved the overdose-reversing drug Narcan for over-the-counter salesIt could take months before Narcan, the brand name for naloxone, is available for purchase without a prescription, the Food and Drug Administration said.
The FDA has approved the overdose-reversing drug Narcan for over-the-counter salesIt could take months before Narcan, the brand name for naloxone, is available for purchase without a prescription, the Food and Drug Administration said.
Consulte Mais informação »
