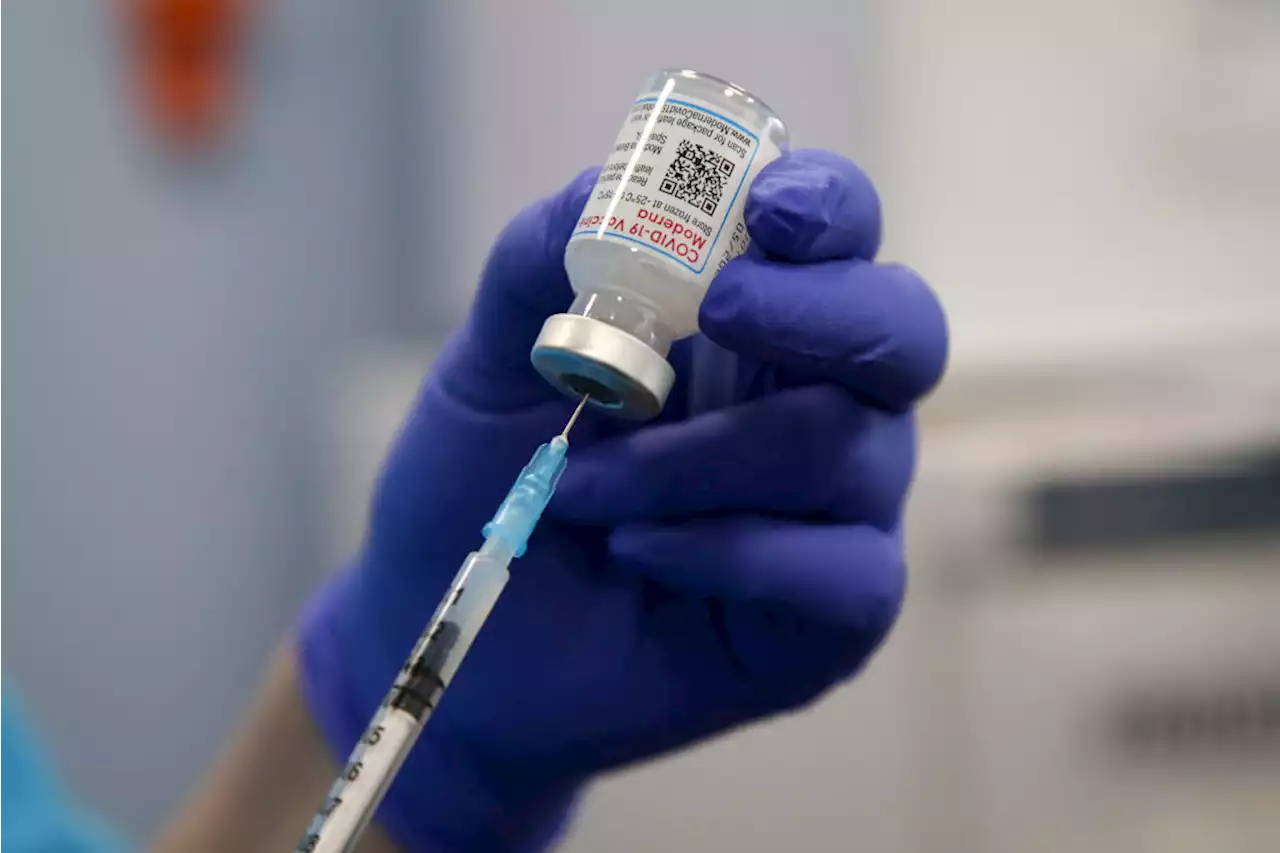
An expert panel recommended that kids have another option when it comes to getting vaccinated against COVID-19
Dinendra Haria/SOPA Images/LightRocket—Getty Imageshe Food and Drug Administration’s advisory committee voted unanimously on June 14 to recommend the emergency-use authorization of Moderna’s COVID-19 vaccine for children ages six to 17 years. If it’s also recommended by the U.S. Centers for Disease Control and Prevention , Moderna’s will be the second COVID-19 vaccine available to this age group.
Moderna’s pediatric vaccine comes in two different doses, depending on children’s ages. Older children ages 12 to 17 would receive the same dose as adults, 100 micrograms per shot. Children ages six to 11 would receive half that dose in each of their two shots; the company’s studies found that the lower dose led to sufficient levels of virus-fighting antibodies and lowered the risk of potential side effects.
Some members of the committee—including Dr. Paul Offit, professor of pediatrics at the Children’s Hospital of Philadelphia—raised concerns that the lower efficacy against Omicron would mean that children, like adults, would need an additional dose to maintain immunity at sufficient levels to protect them against serious disease. “This is a three-dose vaccine if it is to be effective against serious Omicron disease,” he said.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Consulte Mais informação »
 FDA panel backs Moderna's COVID-19 vaccine for kids ages 6-17The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
FDA panel backs Moderna's COVID-19 vaccine for kids ages 6-17The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
Consulte Mais informação »
 FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
Consulte Mais informação »
 FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
Consulte Mais informação »
 FDA: Pfizer COVID-19 Shot Appears Effective For Kids Under 5The review by federal health officials was a key step toward a long-awaited decision to begin vaccinating the youngest American children.
FDA: Pfizer COVID-19 Shot Appears Effective For Kids Under 5The review by federal health officials was a key step toward a long-awaited decision to begin vaccinating the youngest American children.
Consulte Mais informação »