Mrs Miller’s Homemade Jams issued a recall for two jams that might contain undeclared soy: Smokey BBQ Bacon Jam and Spicy Chili Bacon Jam.
Mrs Miller’s Homemade Jams recall
The company found that soy was present in the Worchestire Sauce and Hickory Smoke ingredients that were used to make the Homemade Jams in the recall. that primarily impacted buyers in the state of New York, this jam recall mainly concerns buyers in Ohio.That’s where Mrs Miller’s Homemade Jams sold most of its products, according to the list of retailers in the press release. But the Smokey BBQ Bacon Jam and Spicy Chili Bacon Jam were available in other states, too. And online sales made the product accessible from anywhere in the US.
You may experience severe constriction of the airways, which makes it impossible to breathe. The pulse can increase as people go into shock and the blood pressure drops. Dizziness, lightheadedness, or loss of consciousness are also common with anaphylaxis.The two jams are still fine to eat if you’re not allergic to soy. But keeping products containing undeclared allergens at home might pose a risk to other people in your household, including visitors.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
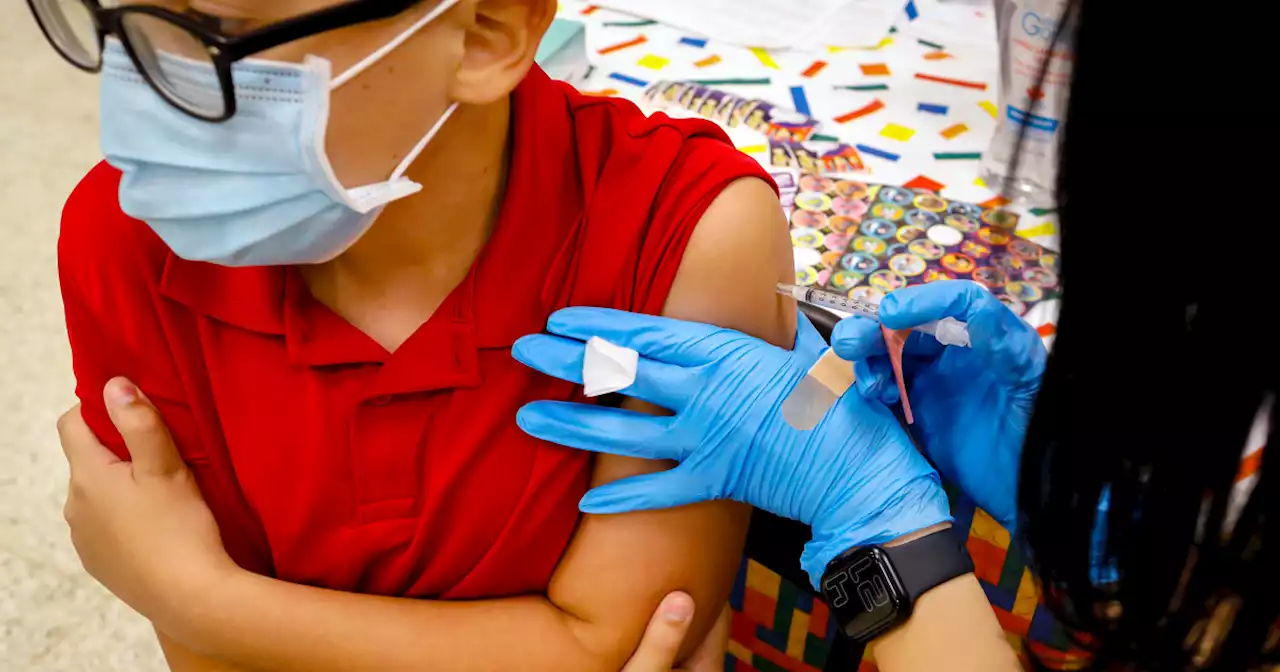 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Consulte Mais informação »
 FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
Consulte Mais informação »
 Abbott reaches agreement with FDA to reopen baby formula plantInfant formula maker Abbott says it's reached an agreement with U.S. health officials to restart production at its largest domestic factory, a key step toward easing a nationwide shortage: abc15
Abbott reaches agreement with FDA to reopen baby formula plantInfant formula maker Abbott says it's reached an agreement with U.S. health officials to restart production at its largest domestic factory, a key step toward easing a nationwide shortage: abc15
Consulte Mais informação »
 Abbott agrees to consent decree with FDA, could restart plant within 2 weeks, pending court approvalThe baby formula manufacturer at the heart of a nationwide recall says it has reached an agreement with the FDA on steps necessary to resume production at its shuttered plant.
Abbott agrees to consent decree with FDA, could restart plant within 2 weeks, pending court approvalThe baby formula manufacturer at the heart of a nationwide recall says it has reached an agreement with the FDA on steps necessary to resume production at its shuttered plant.
Consulte Mais informação »
 Baby formula maker Abbott says agreement reached with FDA to reopen Sturgis, Michigan facilityAfter production resumes, Abbott has said it will take at least eight weeks to begin shipping new product to stores.
Baby formula maker Abbott says agreement reached with FDA to reopen Sturgis, Michigan facilityAfter production resumes, Abbott has said it will take at least eight weeks to begin shipping new product to stores.
Consulte Mais informação »
 FDA, Abbott agree on plan to resume production of infant formula at Michigan plantBREAKING: FDA and Abbott Nutrition agree on plan to resume operations at its infant formula facility in Sturgis, Michigan, the company announces.
FDA, Abbott agree on plan to resume production of infant formula at Michigan plantBREAKING: FDA and Abbott Nutrition agree on plan to resume operations at its infant formula facility in Sturgis, Michigan, the company announces.
Consulte Mais informação »
