FDA pulls authorization of COVID drug MedTwitter
-Eli Lilly and Co's COVID-19 drug bebtelovimab is not currently authorized for emergency use in the United States, the Food and Drug Administration said, citing it is not expected to neutralize the dominant BQ.1 and BQ.1.1 subvariants of Omicron.
AstraZeneca Plc's monoclonal antibody Evusheld is also authorized for protection against COVID-19 infection in some people.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Consulte Mais informação »
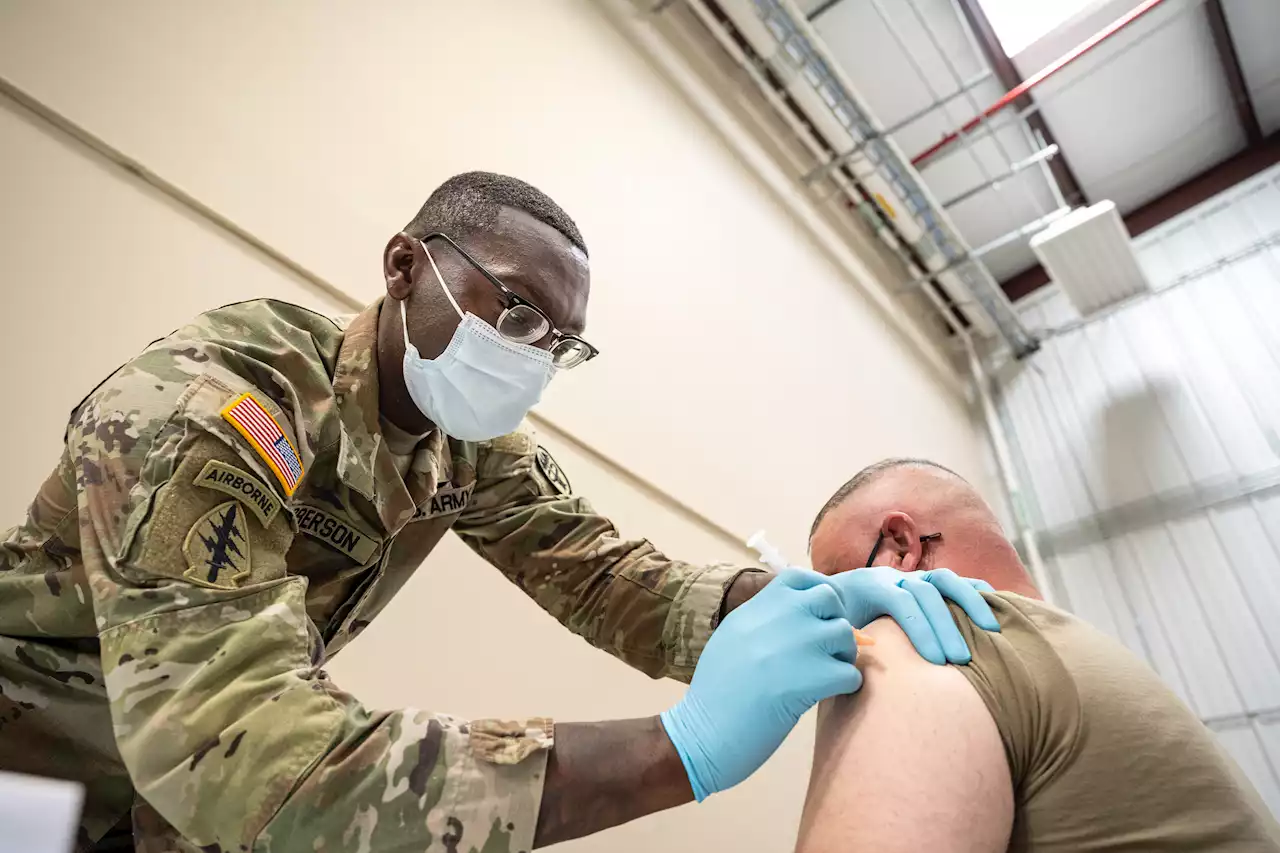 Defense bill could roll back Covid vaccine policy, top Dem saysThousands of troops have been kicked out for refusing the Covid vaccine. Undoing the 2021 policy would be a win for Republicans who argue it's exacerbating a recruiting and retention crisis.
Defense bill could roll back Covid vaccine policy, top Dem saysThousands of troops have been kicked out for refusing the Covid vaccine. Undoing the 2021 policy would be a win for Republicans who argue it's exacerbating a recruiting and retention crisis.
Consulte Mais informação »
 COVID-19 Monoclonal Antibody Treatments No Longer EffectiveThe FDA has said that bebtelovimab, a monoclonal antibody drug given through a vein, is no longer authorized because it is not effective against the leading strains of COVID-19. This drug was the last remaining monoclonal antibody treatment of six that had been authorized for emergency use during the pandemic.
COVID-19 Monoclonal Antibody Treatments No Longer EffectiveThe FDA has said that bebtelovimab, a monoclonal antibody drug given through a vein, is no longer authorized because it is not effective against the leading strains of COVID-19. This drug was the last remaining monoclonal antibody treatment of six that had been authorized for emergency use during the pandemic.
Consulte Mais informação »
 Keep COVID military vaccine mandate, defense chief saysLast year Secretary Lloyd Austin directed that all troops get the vaccine or face potential expulsion from the military.
Keep COVID military vaccine mandate, defense chief saysLast year Secretary Lloyd Austin directed that all troops get the vaccine or face potential expulsion from the military.
Consulte Mais informação »
 As COVID cases, other viruses continue to rise, SoCal inches closer to a mask mandateExperts say the combination of COVID, the flu, and RSV is putting an enormous strain on hospitals.
As COVID cases, other viruses continue to rise, SoCal inches closer to a mask mandateExperts say the combination of COVID, the flu, and RSV is putting an enormous strain on hospitals.
Consulte Mais informação »
