Coronavirus Update: FDA panel set to review protein-based Novavax vaccine
An advisory panel to the U.S. Food and Drug Administration is meeting Tuesday to review the vaccine developed by Novavax Inc. as a potential fourth shot for American adults and will vote on whether the risks outweigh the benefits.
“If causally associated, the risk of myocarditis following NVX-CoV2373 could be higher than reported during post-authorization use of mRNA COVID-19 vaccines,” the FDA said. The meeting comes as U.S. cases are averaging 98,867 a day, down 8% from two weeks ago, according to a New York Times tracker. That’s about four times higher than where they were in early April, but well below numbers seen in January, when omicron was at a peak.
Coronavirus Update: MarketWatch’s daily roundup has been curating and reporting all the latest developments every weekday since the coronavirus pandemic began• Transportation Secretary Pete Buttigieg announced Monday that he has tested positive for COVID, as he confirmed in a tweet. The news comes after Buttigieg traveled to Michigan last week to tout President Joe Biden’s infrastructure law and federal investments in freight rail and supply-chain improvements.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA Advisers Consider Novavax COVID-19 Vaccine This WeekThe FDA’s vaccine advisors are scheduled to meet on Tuesday to consider authorizing the Novavax COVID-19 vaccine. The committee will vote on whether the benefits of the two-dose vaccine outweigh the risks for ages 18 and older.
FDA Advisers Consider Novavax COVID-19 Vaccine This WeekThe FDA’s vaccine advisors are scheduled to meet on Tuesday to consider authorizing the Novavax COVID-19 vaccine. The committee will vote on whether the benefits of the two-dose vaccine outweigh the risks for ages 18 and older.
Consulte Mais informação »
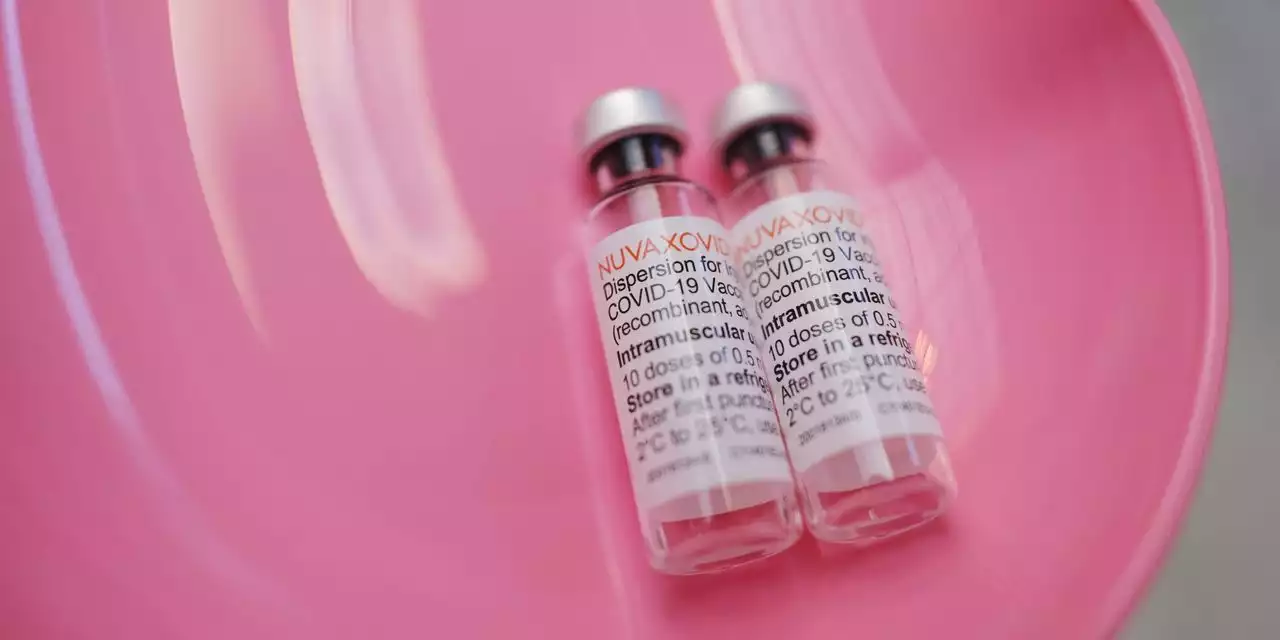 Novavax Covid-19 Vaccine Faces FDA Advisers’ ScrutinyThe outside panel of experts will vote on whether the shot should be authorized, after the agency found it effective but flagged a safety concern.
Novavax Covid-19 Vaccine Faces FDA Advisers’ ScrutinyThe outside panel of experts will vote on whether the shot should be authorized, after the agency found it effective but flagged a safety concern.
Consulte Mais informação »
 Novavax Faces High Stakes FDA Review This Week That Will Decide the Fate of Its Covid Vaccine in the U.S.The Food and Drug Administration’s independent experts will review the safety and effectiveness of Novavax’s Covid vaccine Tuesday. If they endorse the vaccine, the FDA will almost certainly authorize the shot for adults in the U.S. Novavax’s vaccine uses protein technology that is different Pfizer and Moderna’s messenger RNA shots. Novavax was part of the U.S. race to develop a…
Novavax Faces High Stakes FDA Review This Week That Will Decide the Fate of Its Covid Vaccine in the U.S.The Food and Drug Administration’s independent experts will review the safety and effectiveness of Novavax’s Covid vaccine Tuesday. If they endorse the vaccine, the FDA will almost certainly authorize the shot for adults in the U.S. Novavax’s vaccine uses protein technology that is different Pfizer and Moderna’s messenger RNA shots. Novavax was part of the U.S. race to develop a…
Consulte Mais informação »
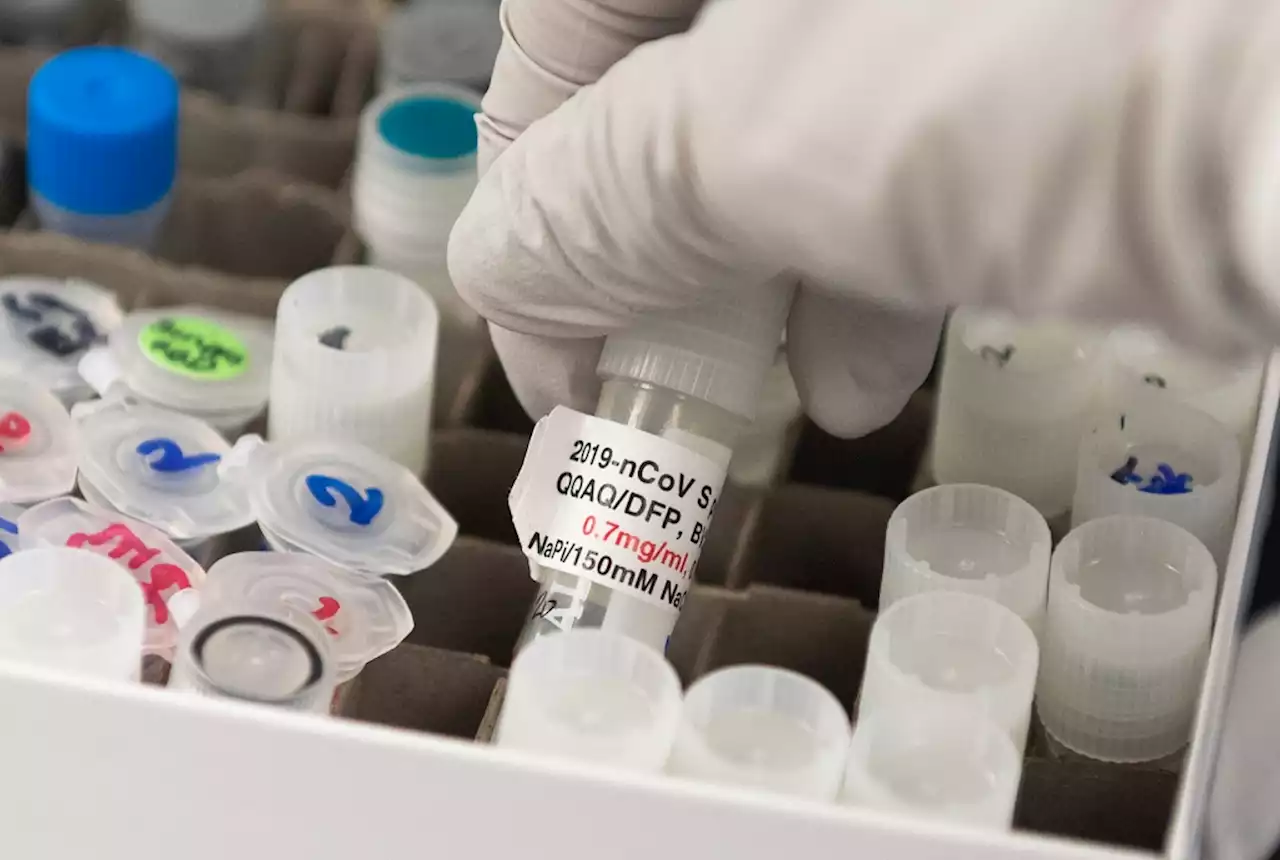 Can new COVID vaccine win over the anti-vaxxers?Designed using traditional approach, Novavax vaccine gets review by FDA advisory panel on Tuesday.
Can new COVID vaccine win over the anti-vaxxers?Designed using traditional approach, Novavax vaccine gets review by FDA advisory panel on Tuesday.
Consulte Mais informação »
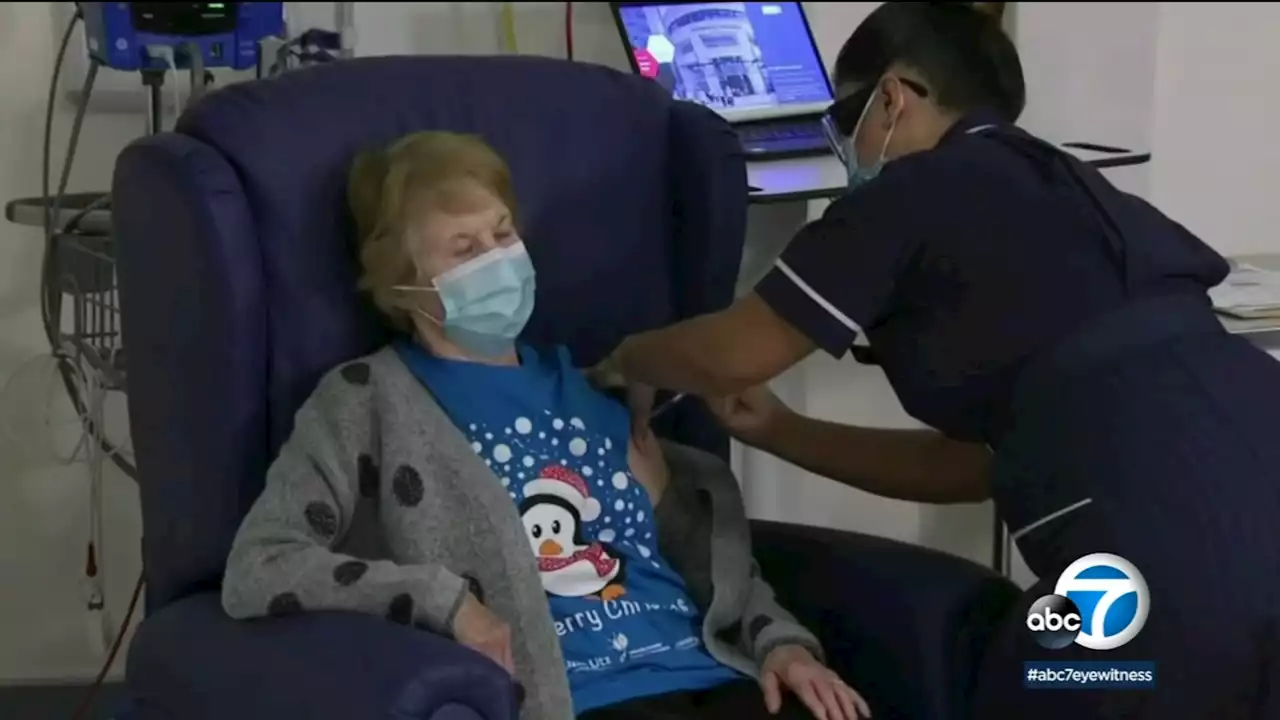 Novavax hoping its COVID-19 vaccine using traditional technology wins over vaccine skepticsThe Novavax COVID-19 vaccine is made with an old school technology, but will it be enough to win over vaccine skeptics?
Novavax hoping its COVID-19 vaccine using traditional technology wins over vaccine skepticsThe Novavax COVID-19 vaccine is made with an old school technology, but will it be enough to win over vaccine skeptics?
Consulte Mais informação »
 FDA Advisers Consider Novavax COVID-19 Vaccine This WeekThe FDA’s vaccine advisors are scheduled to meet on Tuesday to consider authorizing the Novavax COVID-19 vaccine. The committee will vote on whether the benefits of the two-dose vaccine outweigh the risks for ages 18 and older.
FDA Advisers Consider Novavax COVID-19 Vaccine This WeekThe FDA’s vaccine advisors are scheduled to meet on Tuesday to consider authorizing the Novavax COVID-19 vaccine. The committee will vote on whether the benefits of the two-dose vaccine outweigh the risks for ages 18 and older.
Consulte Mais informação »
