The meetings are a sign that the vaccines are moving closer to a possible authorization.
During the winter omicron wave, children under age 5 were hospitalized with Covid at five times the rate of the peak when the delta variant was predominant, according to the Centers for Disease Control and Prevention. About 75% of children in the U.S. have been infected by the virus at some point during the pandemic, according to data from national blood sample survey from the CDC.
Novavax was an early participant in Operation Warp Speed, the U.S. government's race to develop a vaccine against Covid in 2020. However, Moderna and Pfizer ultimately beat Novavax to the punch because the company struggled with manufacturing issues. Novavax produces the virus spike outside the human body. The genetic code for the spike is put into a baculovirus that infects insect cells, which then produce copies of the spike that are purified and extracted for the shots. The vaccine also uses an adjuvant, an extract purified from the bark of a tree in South America, to induce a broader immune response.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Lawmakers scrutinize McKinsey's opioid, FDA consulting workLawmakers vowed to continue investigating consulting firm McKinsey after a hearing scrutinizing its work for the Food and Drug Administration even as it advised opioid makers on boosting sales
Lawmakers scrutinize McKinsey's opioid, FDA consulting workLawmakers vowed to continue investigating consulting firm McKinsey after a hearing scrutinizing its work for the Food and Drug Administration even as it advised opioid makers on boosting sales
Consulte Mais informação »
 Pfizer Asks FDA to Authorize COVID Booster Shots for Ages 5-11Pfizer and BioNTech have applied to the FDA for authorization of their COVID-19 booster shot for children ages 5-11, according to an update from Pfizer.
Pfizer Asks FDA to Authorize COVID Booster Shots for Ages 5-11Pfizer and BioNTech have applied to the FDA for authorization of their COVID-19 booster shot for children ages 5-11, according to an update from Pfizer.
Consulte Mais informação »
 Lawmakers grill consulting company over work with opioid manufacturers and FDA at same timeA global consulting company is under fire for accusations it helped opioid manufacturers make big profits while also consulting for the government agency in charge of regulating those very drugs.
Lawmakers grill consulting company over work with opioid manufacturers and FDA at same timeA global consulting company is under fire for accusations it helped opioid manufacturers make big profits while also consulting for the government agency in charge of regulating those very drugs.
Consulte Mais informação »
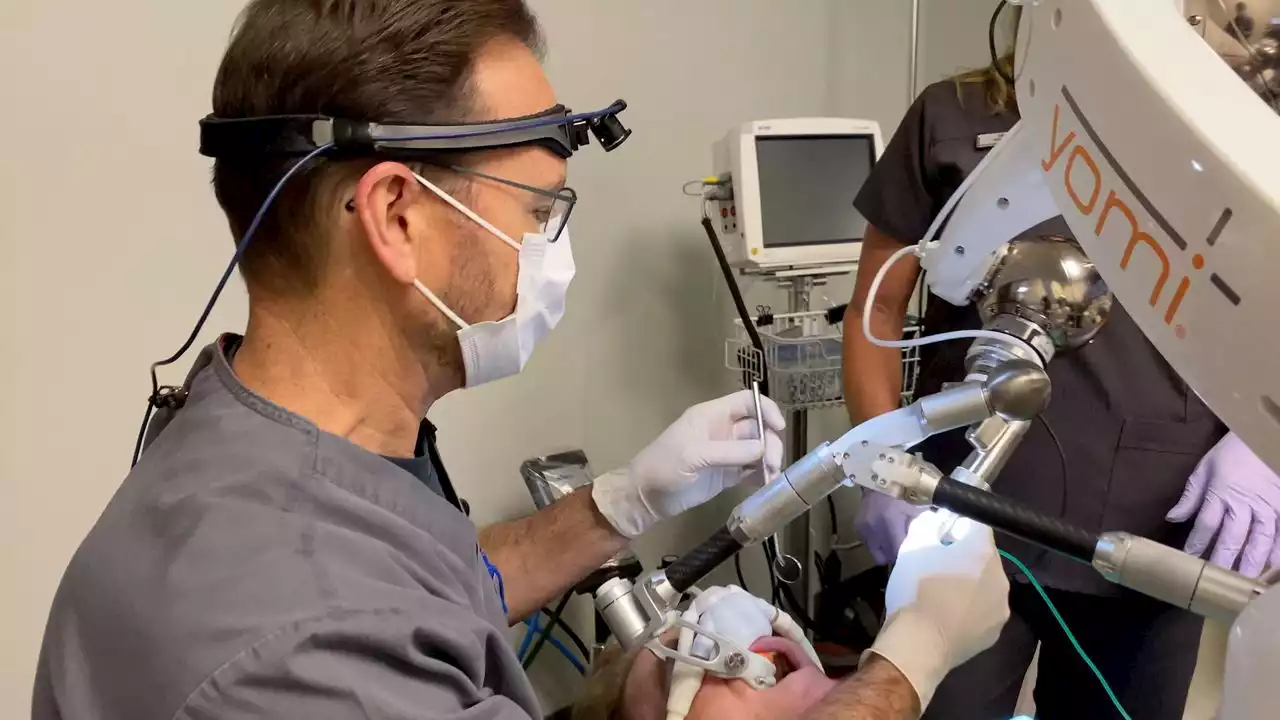 North Texas dentist uses first-ever FDA-cleared dental robotDr. J. Darrell Steele stays up-to-date with the latest technology in dentistry. When he learned about a robotic device cleared by the FDA as the first robot-assisted dental surgical system used for a single-tooth implant, he was interested.
North Texas dentist uses first-ever FDA-cleared dental robotDr. J. Darrell Steele stays up-to-date with the latest technology in dentistry. When he learned about a robotic device cleared by the FDA as the first robot-assisted dental surgical system used for a single-tooth implant, he was interested.
Consulte Mais informação »
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Consulte Mais informação »
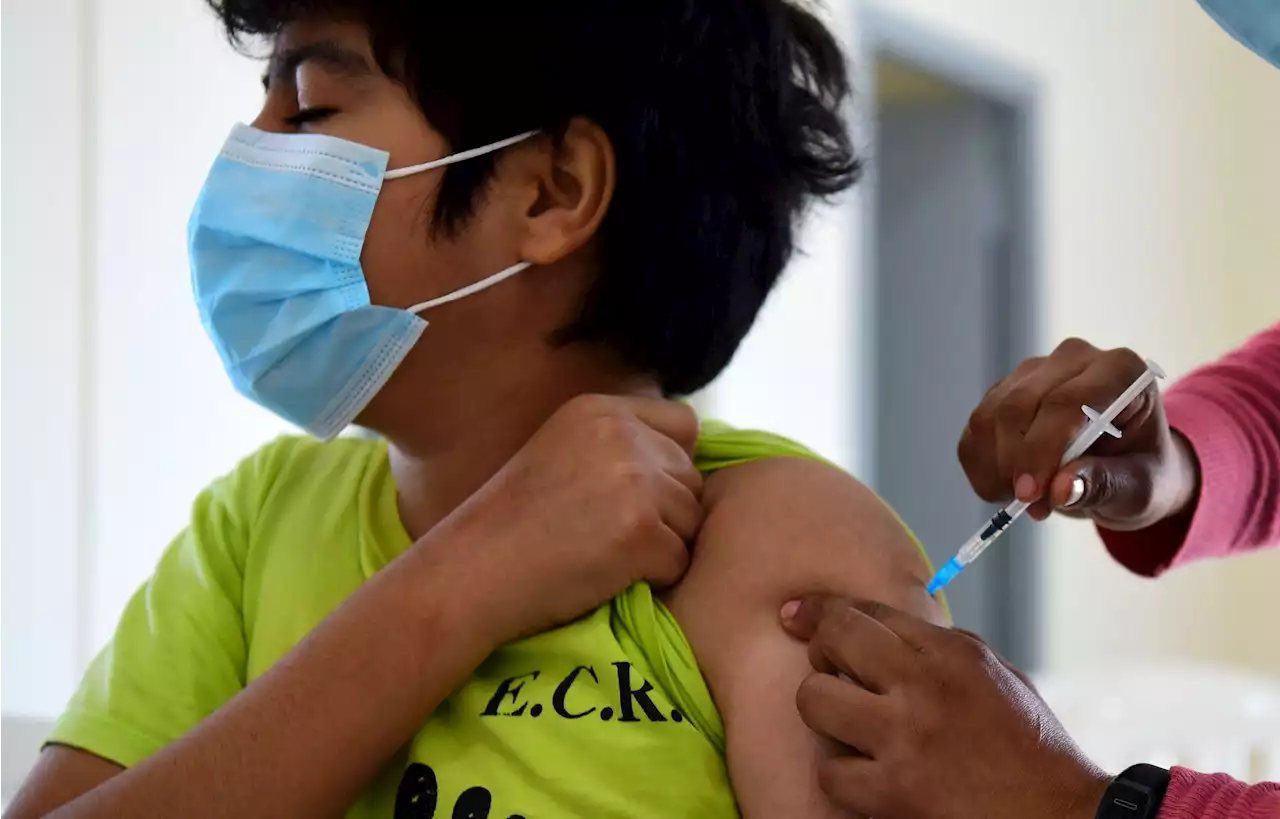 Pfizer Asks FDA To Authorize Covid Booster For Kids 5-11As the omicron variant puts children at greater risk of hospitalization, public health officials have worked to make Covid-19 treatments more accessible to young people.
Pfizer Asks FDA To Authorize Covid Booster For Kids 5-11As the omicron variant puts children at greater risk of hospitalization, public health officials have worked to make Covid-19 treatments more accessible to young people.
Consulte Mais informação »
