The overdose-reversal drug naloxone should be available as an over-the-counter medication to aid the national response to the opioid crisis, U.S. health...
WASHINGTON — The overdose-reversal drug naloxone should be available as an over-the-counter medication to aid the national response to the opioid crisis, U.S. health advisers said Wednesday.
Panel members urged the FDA to move swiftly rather than waiting for Emergent to conduct a follow-up study with the easier-to-understand label. The potential move represents the latest government effort to increase use of a medication that has been a key tool in the battle against the U.S. overdose epidemic that kills more than 100,000 people annually. The decades-old drug can counteract the effects of an opioid overdose in minutes.
FDA staff members flagged also cautioned that a number of participants had trouble following the directions, in part because of the way the multistep instructions were laid out across two sides of the carton, FDA noted. Community advocates and organizations that favor distributing the drug welcomed the potential approval of an over-the-counter version.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA Panel Backs Moving Opioid Antidote Narcan Over the CounterU.S. health advisers say the overdose-reversal drug naloxone should be made available as an over-the-counter medication. It’s the latest government proposal to increase use of the medication against the opioid overdose crisis. The nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations. The positive vote by an Food and Drug Administration advisory panel came despite difficulties understanding how to use the drug in a company study. FDA will make a final decision on the drug in coming weeks.
FDA Panel Backs Moving Opioid Antidote Narcan Over the CounterU.S. health advisers say the overdose-reversal drug naloxone should be made available as an over-the-counter medication. It’s the latest government proposal to increase use of the medication against the opioid overdose crisis. The nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations. The positive vote by an Food and Drug Administration advisory panel came despite difficulties understanding how to use the drug in a company study. FDA will make a final decision on the drug in coming weeks.
Consulte Mais informação »
 FDA panel backs moving opioid antidote Narcan over the counterThe nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations.
FDA panel backs moving opioid antidote Narcan over the counterThe nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations.
Consulte Mais informação »
 FDA considers making Narcan opioid overdose antidote available without prescription | CNNAdvisers to the US Food and Drug Administration will meet Wednesday to discuss whether a nasal spray version of the opioid overdose antidote Narcan should be made available over-the-counter.
FDA considers making Narcan opioid overdose antidote available without prescription | CNNAdvisers to the US Food and Drug Administration will meet Wednesday to discuss whether a nasal spray version of the opioid overdose antidote Narcan should be made available over-the-counter.
Consulte Mais informação »
 Narcan overdose antidote close to being sold over the counter: FDA meetingHealth experts say a higher accessibility to the drug could help combat the current opioid crisis in the US
Narcan overdose antidote close to being sold over the counter: FDA meetingHealth experts say a higher accessibility to the drug could help combat the current opioid crisis in the US
Consulte Mais informação »
 FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone should be made available as an over-the-counter medication
FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone should be made available as an over-the-counter medication
Consulte Mais informação »
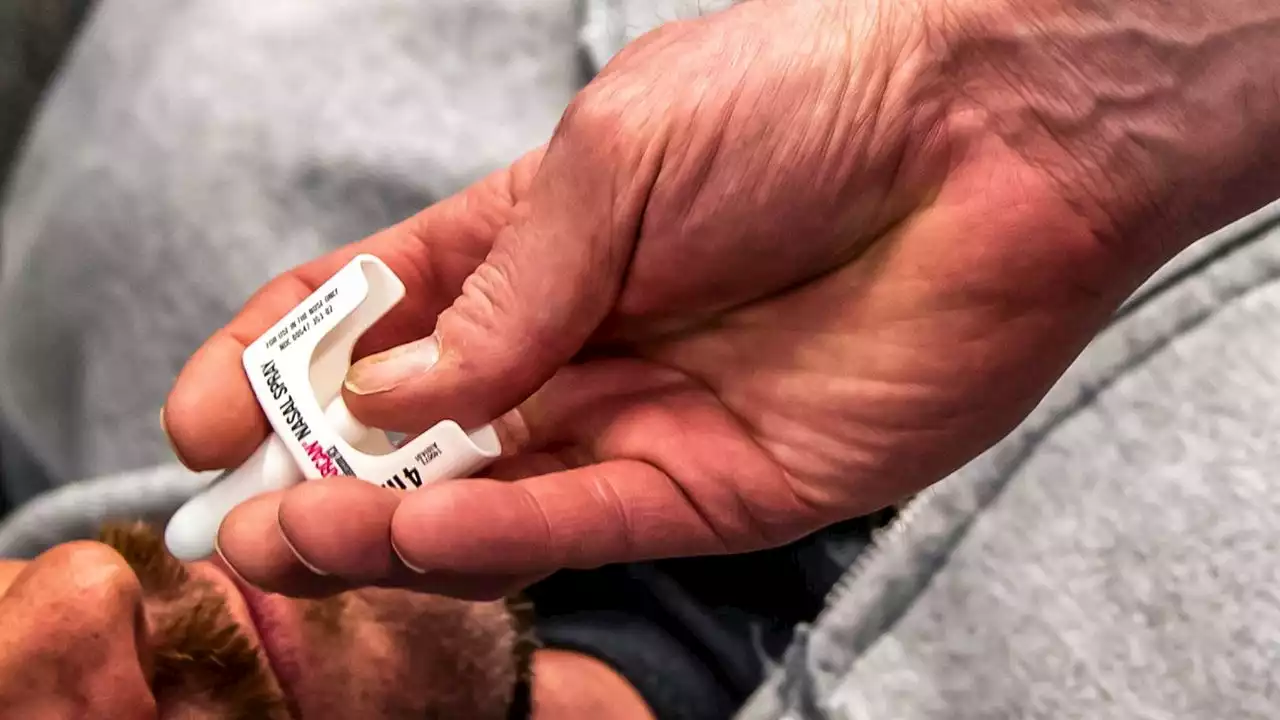 FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone, commonly referred to as Narcan, should be made available as an over-the-counter medication.
FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone, commonly referred to as Narcan, should be made available as an over-the-counter medication.
Consulte Mais informação »
