U.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds.
The Food and Drug Administration’s decision opens a fourth dose of the Pfizer or Moderna vaccines to those people at least four months after their previous booster.
The move comes at a time of great uncertainty. COVID-19 cases have dropped to low levels after the winter surge of the super-contagious omicron variant. Two vaccine doses plus a booster still provide strong protection against severe disease and death, CDC data show. There’s limited evidence to tell how much benefit another booster could offer right now. FDA made the decision without input from its independent panel of experts that has wrestled with how much data is required to expand shots.
None of the COVID-19 vaccines are as strong against the omicron mutant as they were against earlier versions of the virus. Also, protection against milder infections naturally wanes over time. But the immune system builds multiple layers of defense and the type that prevents severe illness and death is holding up.
What’s far from clear is how long any extra benefit from another booster would last, and thus when to get it. “If you get a booster too close together, it’s not doing any harm — you’re just not going to get much benefit from it,” said Wherry.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Covid-19: Two Covid-related deaths and 1,172 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,301.
Covid-19: Two Covid-related deaths and 1,172 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,301.
Consulte Mais informação »
 Covid-19: Five Covid-related deaths and 1,204 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,306.
Covid-19: Five Covid-related deaths and 1,204 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,306.
Consulte Mais informação »
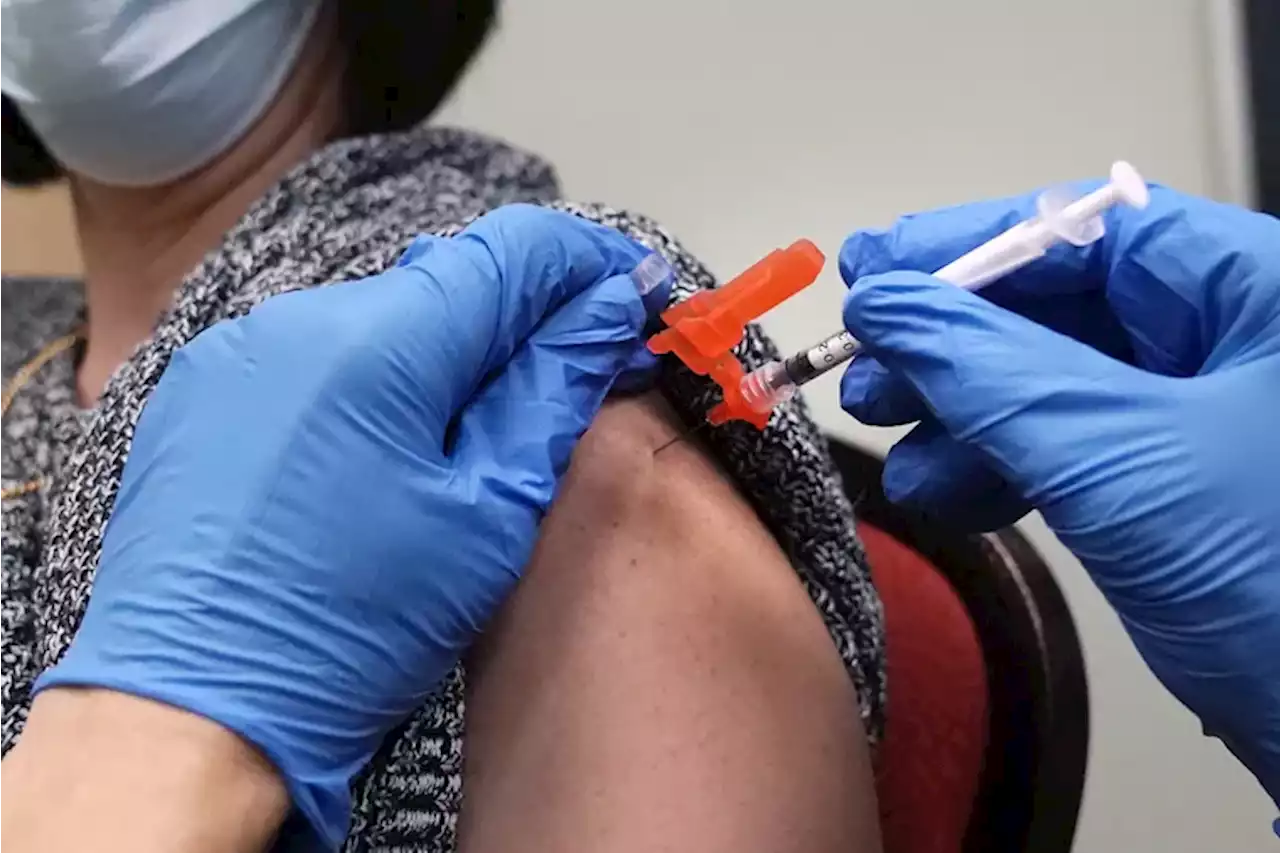 The FDA is expected to clear an additional COVID-19 booster shotThe FDA is poised to clear a fourth dose of the mRNA coronavirus vaccine for adults age 50 and older, looking to shore up protections for more vulnerable groups, a person familiar with the matter said.
The FDA is expected to clear an additional COVID-19 booster shotThe FDA is poised to clear a fourth dose of the mRNA coronavirus vaccine for adults age 50 and older, looking to shore up protections for more vulnerable groups, a person familiar with the matter said.
Consulte Mais informação »
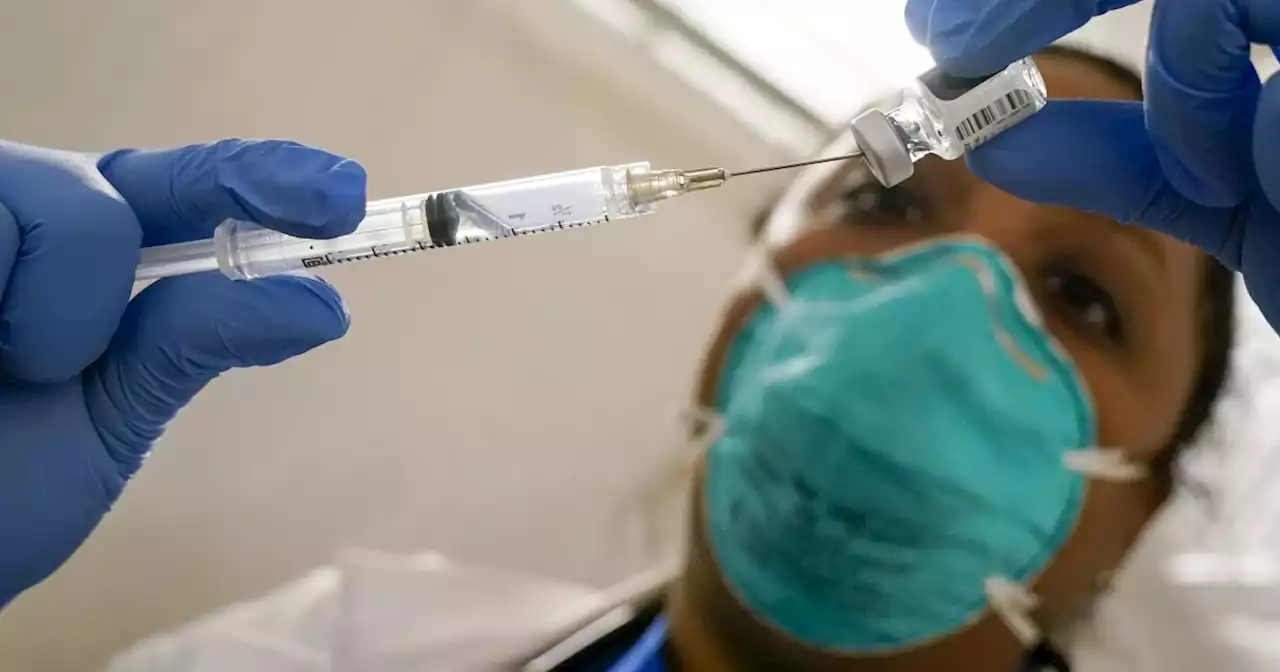 The FDA is expected to approve a second COVID booster for people 50 and upThe FDA will likely authorize a secondary booster dose for adults 50 years and older this week.
The FDA is expected to approve a second COVID booster for people 50 and upThe FDA will likely authorize a secondary booster dose for adults 50 years and older this week.
Consulte Mais informação »