FDA has approved the first treatment for Friedreich's ataxia
The US Food and Drug Administration has approved the first treatment for the neurodegenerative disorder Friedreich's ataxia for use in adults and adolescents aged 16 and older.
"Friedreich's ataxia is a debilitating neuromuscular disease that progressively robs patients of their mobility and independence," Susan Perlman, MD, Department of Neurology, David Geffen School of Medicine at UCLA, said in the release. Previous studies have demonstrated that nuclear factor erythroid-derived 2-related factor 2 signaling is grossly impaired in patients with the disorder. Omaveloxolone is an activator of the protein Nrf2 that protects against inflammation.
Treatment with the novel medication led to statistically significant lower mFARS scores, signifying less impairment, relative to placebo at week 48. The placebo-corrected difference between the two groups was -2.41 points .
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
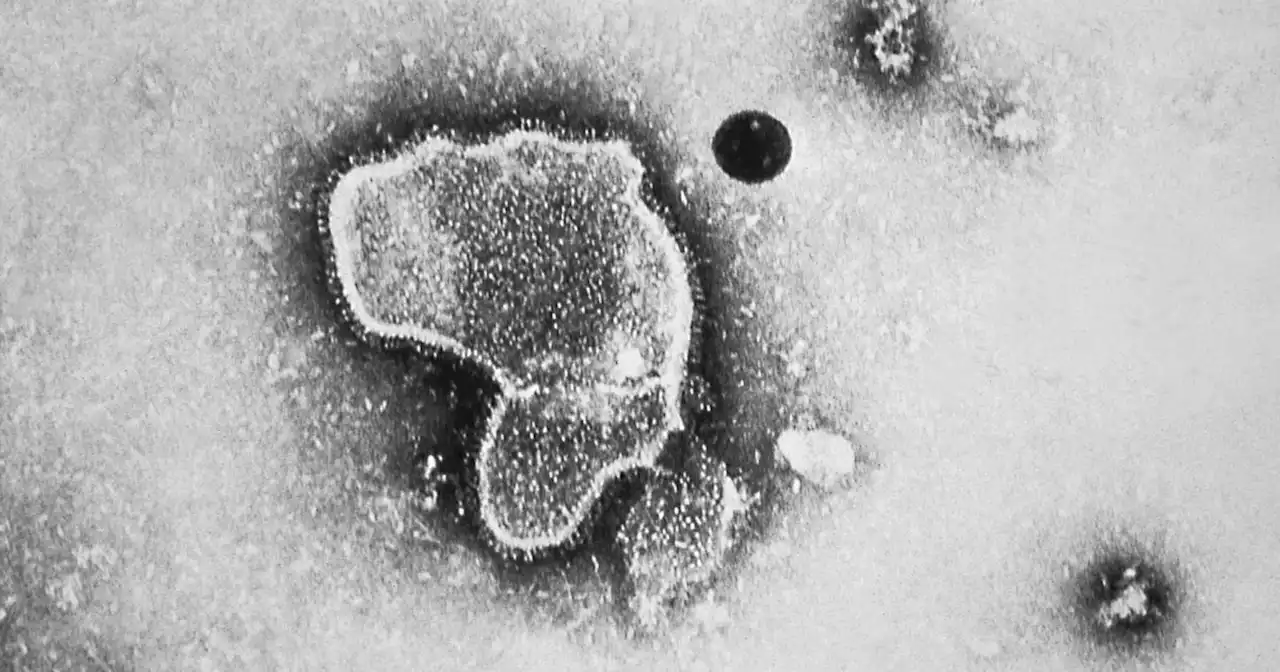 Paving the way for the world's first RSV vaccine, FDA advisers recommend shot from PfizerBREAKING: FDA advisers recommend that the agency approve the country’s first RSV vaccine for older people, a shot from Pfizer for adults ages 60 and up.
Paving the way for the world's first RSV vaccine, FDA advisers recommend shot from PfizerBREAKING: FDA advisers recommend that the agency approve the country’s first RSV vaccine for older people, a shot from Pfizer for adults ages 60 and up.
Consulte Mais informação »
 FDA Advisors Recommend First-Ever RSV Vaccine From Pfizer, Despite Possible Guillain-Barre RisksSeveral FDA advisors said there could be a significant safety issue after two vaccine recipients out of about 20,000 developed Guillain-Barre syndrome.
FDA Advisors Recommend First-Ever RSV Vaccine From Pfizer, Despite Possible Guillain-Barre RisksSeveral FDA advisors said there could be a significant safety issue after two vaccine recipients out of about 20,000 developed Guillain-Barre syndrome.
Consulte Mais informação »
 FDA Considers Two Drugmakers for First RSV VaccineAn FDA advisory panel is considering two drugmakers to make the first respiratory syncytial virus (RSV) vaccine. Pfizer and GSK seek to lead the market that could be worth between $5 billion and $10 billion.
FDA Considers Two Drugmakers for First RSV VaccineAn FDA advisory panel is considering two drugmakers to make the first respiratory syncytial virus (RSV) vaccine. Pfizer and GSK seek to lead the market that could be worth between $5 billion and $10 billion.
Consulte Mais informação »
 FDA Approves First Biologic Treatment for Polymyalgia RheumaticaThe interleukin-6 receptor antagonist sarilumab (Kevzara) will be indicated in adults who have had an inadequate response to corticosteroids or could not tolerate a corticosteroid taper.
FDA Approves First Biologic Treatment for Polymyalgia RheumaticaThe interleukin-6 receptor antagonist sarilumab (Kevzara) will be indicated in adults who have had an inadequate response to corticosteroids or could not tolerate a corticosteroid taper.
Consulte Mais informação »
