The covid vaccines are expected to be available later this week after the Centers for Disease Control and Prevention signs off.
“We are seeing more people with covid, especially mild covid, but I would not call it a surge,” said Shira I. Doron, chief of infection control at Tufts Health and hospital epidemiologist at Tufts Medical Center in Boston.
Hotez recommends that people of all ages get the shot. “All the covid numbers are climbing, and it is a big unknown: Is this going to turn into a significant wave as we progress into the fall or not?” he said.That’s similar to the FDA position, which argues the coronavirus vaccine should be treated like the flu shot, with an updated product recommended yearly for everyone 6 months and older.
The newly formulated vaccine is a monovalent, with a single component designed to target an omicron variant called XBB.1.5. Previous boosters, which were bivalents, aimed to counter the original coronavirus strain and the BA.4 and BA.5 variants — all long gone.in the United States, but it is closely related to the other XBB variants making up most cases now. That includes
BA.2.86 “appears to be a nothingburger,” said John P. Moore, professor of microbiology and immunology at Weill Cornell Medicine. “It has been showing a very slow rate of increase, not an explosive event. If it were going to have a huge impact, we would know it by now.”that “early research data from multiple labs are reassuring and show that existing antibodies work against the new BA.2.86 variant.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 San Diego's Crinetics releases solid results for drug that treats deadly rare diseaseIf approved by the FDA, Paltusotine would be the company's first drug on the market. It treats a rare disorder caused by a tumor on a person's pituitary gland that causes the release of too much growth hormone.
San Diego's Crinetics releases solid results for drug that treats deadly rare diseaseIf approved by the FDA, Paltusotine would be the company's first drug on the market. It treats a rare disorder caused by a tumor on a person's pituitary gland that causes the release of too much growth hormone.
Consulte Mais informação »
 How to protect your kids and family from Lyme Disease and other tick-borne illnessesAs we move from summer to fall, ticks are going to remain active. Now is the time to learn what you can do to protect your family and pets.
How to protect your kids and family from Lyme Disease and other tick-borne illnessesAs we move from summer to fall, ticks are going to remain active. Now is the time to learn what you can do to protect your family and pets.
Consulte Mais informação »
 SA germ-zapping robot-maker Xenex clears major FDA hurdleThe U.S. Food and Drug Administration has granted Xenex Disinfection Services a De Novo authorization for its LightStrike+ device.
SA germ-zapping robot-maker Xenex clears major FDA hurdleThe U.S. Food and Drug Administration has granted Xenex Disinfection Services a De Novo authorization for its LightStrike+ device.
Consulte Mais informação »
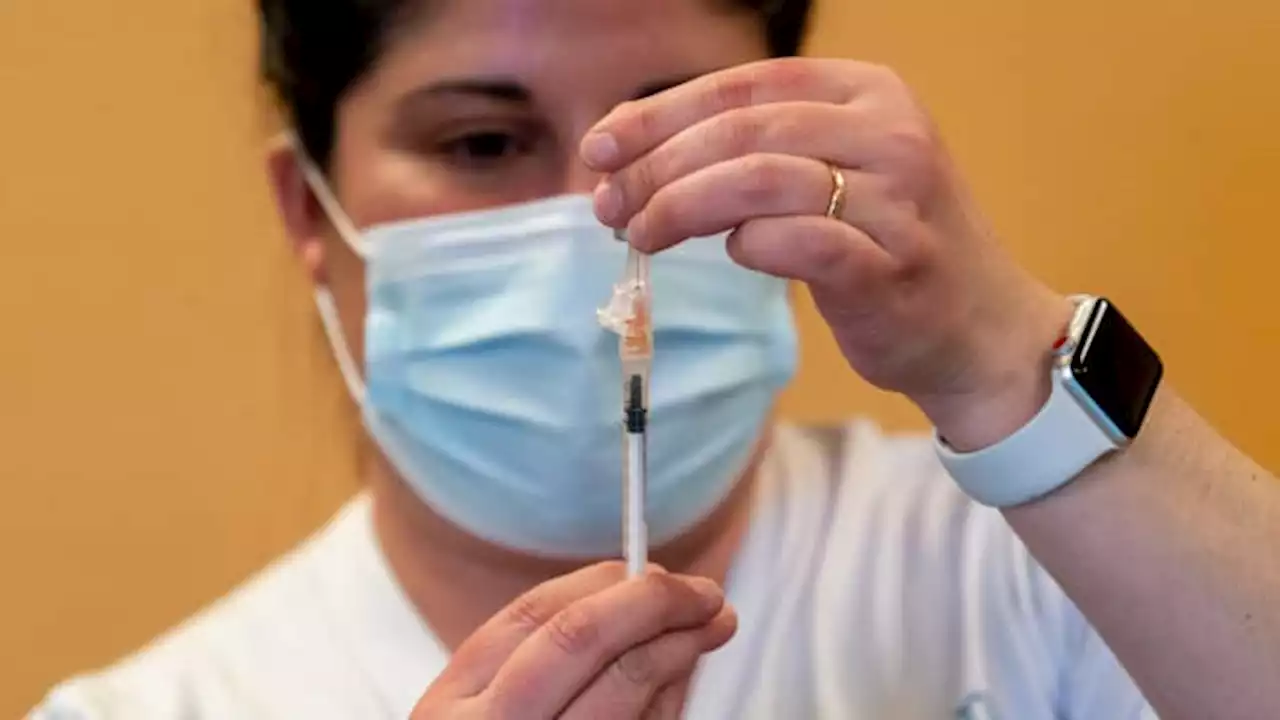 FDA approves updated Covid vaccines from Pfizer and Moderna as hospitalizations riseThe Biden administration said it expects the new Pfizer and Moderna vaccines targeting the omicron variant XBB.1.5 to be available in mid-September.
FDA approves updated Covid vaccines from Pfizer and Moderna as hospitalizations riseThe Biden administration said it expects the new Pfizer and Moderna vaccines targeting the omicron variant XBB.1.5 to be available in mid-September.
Consulte Mais informação »
