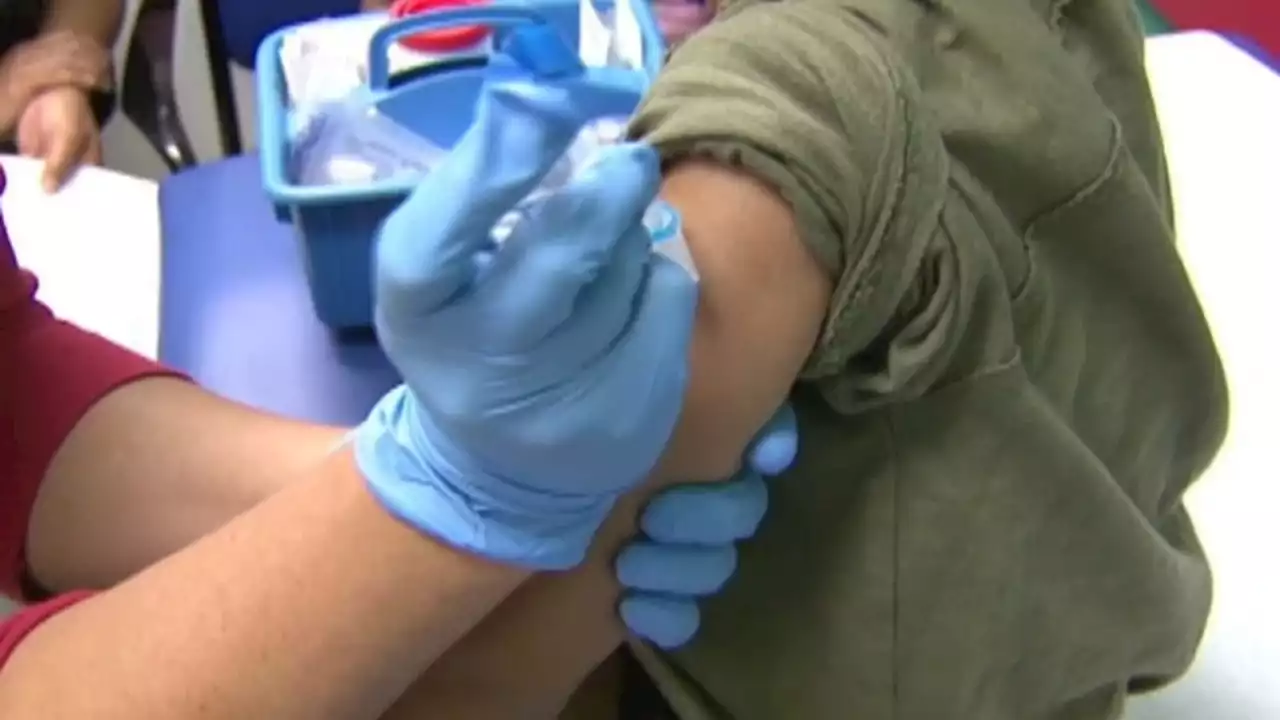
The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
The US Food and Drug Administration announced Friday that it is postponing the meeting of its Vaccines and Related Biological Products Advisory Committee, originally scheduled for February 15, as"new data have recently emerged" regarding Pfizer and BioNTech's emergency use authorization request for their Covid-19 vaccine for children younger than 5.
"This will give the agency time to consider the additional data, allowing for a transparent public discussion as part of our usual scientific and regulatory processes for COVID-19 vaccines. We will provide an update on timing for the advisory committee meeting once we receive additional data on a third dose in this age group from the company's ongoing clinical trial and have an opportunity to complete an updated evaluation," acting FDA Commissioner Dr. Janet Woodcock and Dr.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
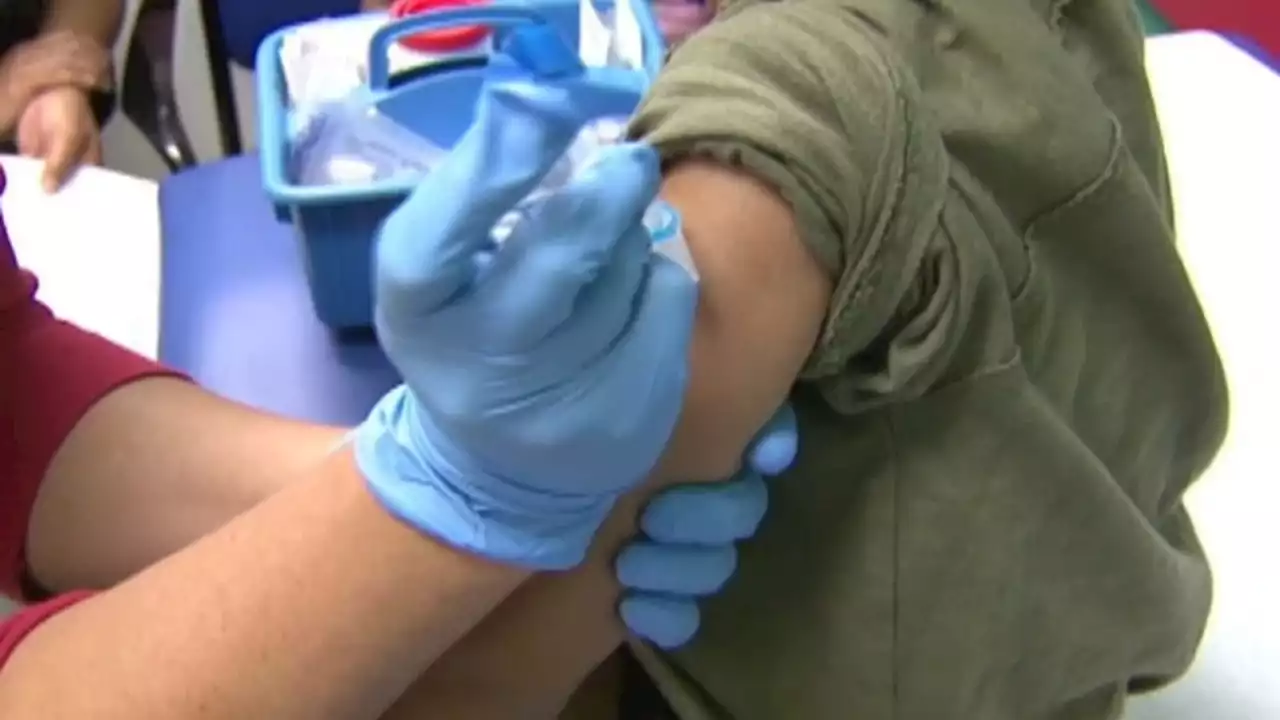 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataBREAKING: The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataBREAKING: The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Consulte Mais informação »
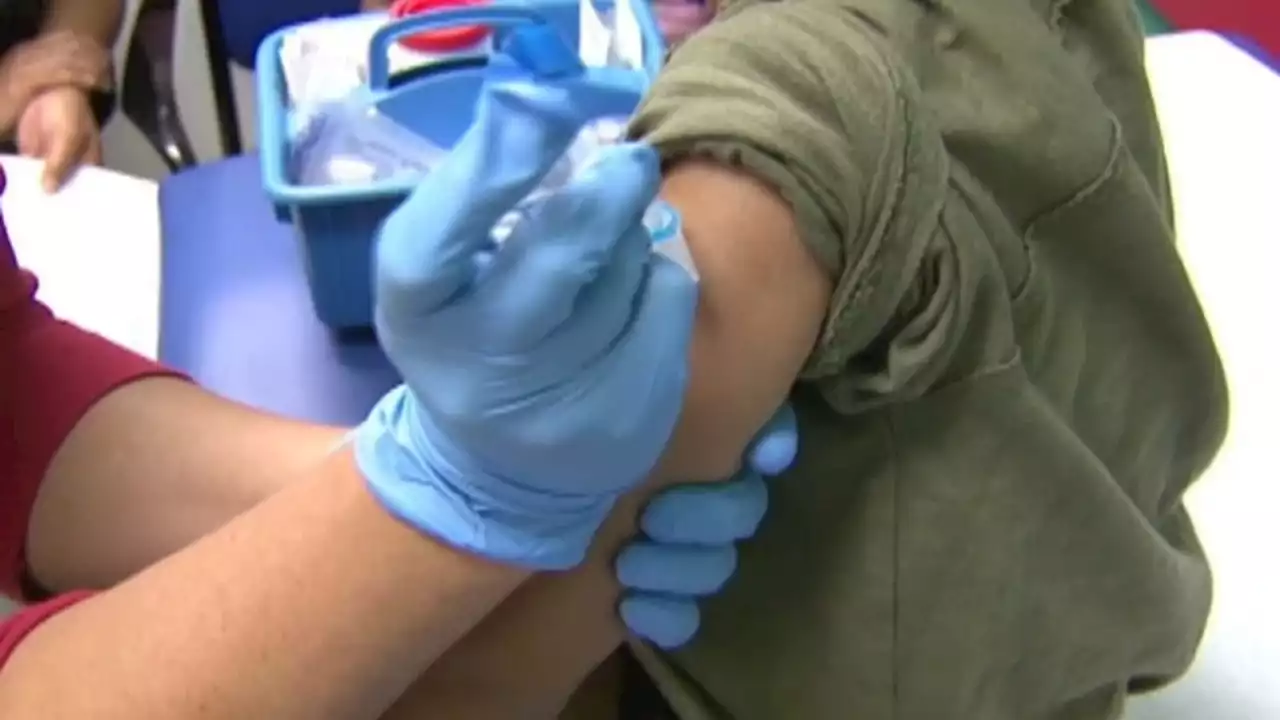 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Consulte Mais informação »
 US preps 10 million doses of COVID-19 vaccine for kids under 5, pending FDA authorizationCOVID-19 vaccines for kids under 5 have not yet been authorized, but the rollout plan is already in the works.
US preps 10 million doses of COVID-19 vaccine for kids under 5, pending FDA authorizationCOVID-19 vaccines for kids under 5 have not yet been authorized, but the rollout plan is already in the works.
Consulte Mais informação »
 In U.K.’s Omicron Wave, Many People Are Dying With Covid-19, Not From ItThe number of people dying in Britain with a recent positive Covid-19 test is significantly overstating the true death toll from the virus for the first time, according to new data.
In U.K.’s Omicron Wave, Many People Are Dying With Covid-19, Not From ItThe number of people dying in Britain with a recent positive Covid-19 test is significantly overstating the true death toll from the virus for the first time, according to new data.
Consulte Mais informação »
 Oscars: COVID-19 Vaccination Will Not Be Required for In-Person Attendees (Exclusive)The Oscars will be back at the Dolby Theatre on March 27, less than a year after the pandemic forced Hollywood’s biggest night to be held as a scaled-down affair at Los Angeles’ Union Station with strict COVID testing requirements. In the time since, safe and free COVID vaccines have become widely available and mandated […]
Oscars: COVID-19 Vaccination Will Not Be Required for In-Person Attendees (Exclusive)The Oscars will be back at the Dolby Theatre on March 27, less than a year after the pandemic forced Hollywood’s biggest night to be held as a scaled-down affair at Los Angeles’ Union Station with strict COVID testing requirements. In the time since, safe and free COVID vaccines have become widely available and mandated […]
Consulte Mais informação »