The FDA has authorized use of the tweaked shots starting at age 6 months, but just who is eligible depends on how many vaccinations they’ve already had. FOX13
Peter Chin-Hong, MD, a professor in the UCSF Health Division of Infectious Diseases, speaks with LiveNOW's Andrew Craft about tri-demic concerns regarding COVID-19, RSV and the flu. Dr. Chin-Hong also speaks about the shortage of children's medications across the U.S., and what parents can do if they are in need of medicine that they cannot find.
Omicron-targeted booster shots made by Moderna and rival Pfizer already were open to everyone 5 and older.The FDA now has authorized use of the tweaked shots starting at age 6 months -- but just who is eligible depends on how many vaccinations they’ve already had, and which kind. Only about 5% of youngsters under age 5 have gotten the full primary series since vaccinations for the littlest kids began in June.
--Pfizer’s vaccine requires three initial doses for tots under age 5 -- and those who haven’t finished that vaccination series will get the original formula for the first two shots and the omicron-targeted version for their third shot. But children under 5 who already got all three Pfizer doses aren’t yet eligible for an updated booster.The FDA expects data from Pfizer and its partner BioNTech sometime next month to determine whether those tots will need an omicron-targeted booster "and we will act on that as soon as we can," he said.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
Consulte Mais informação »
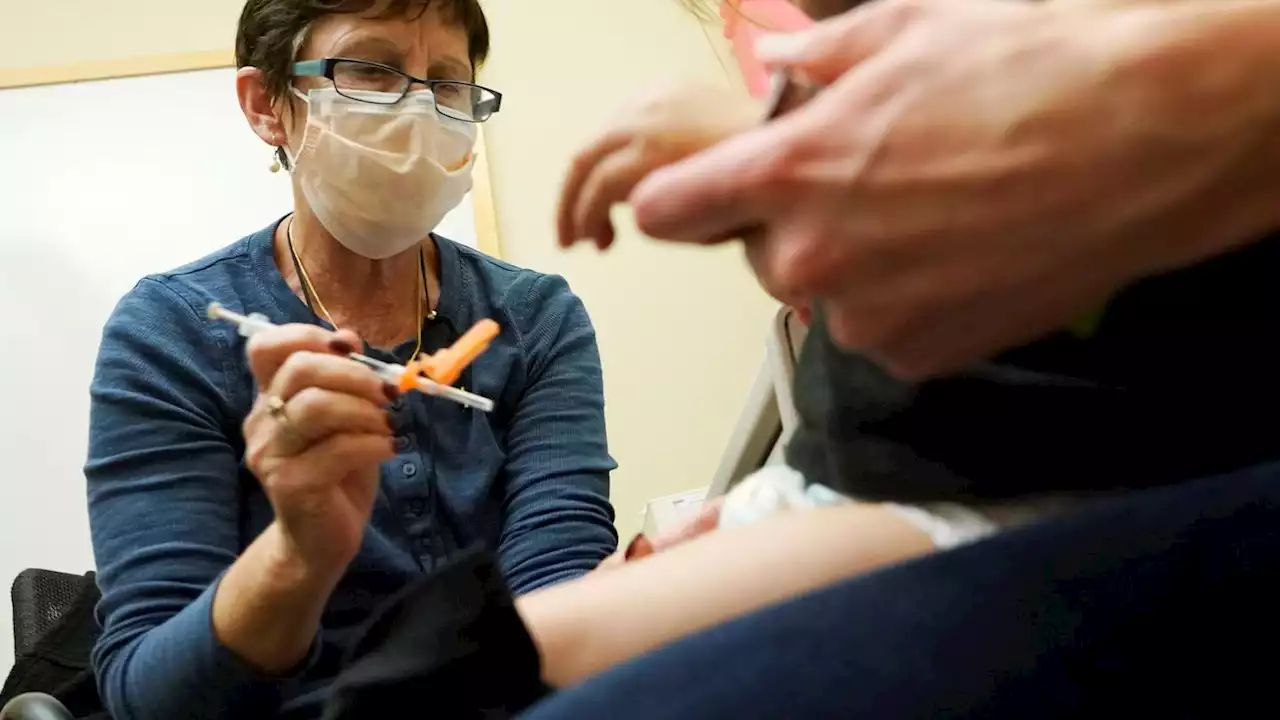 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
Consulte Mais informação »
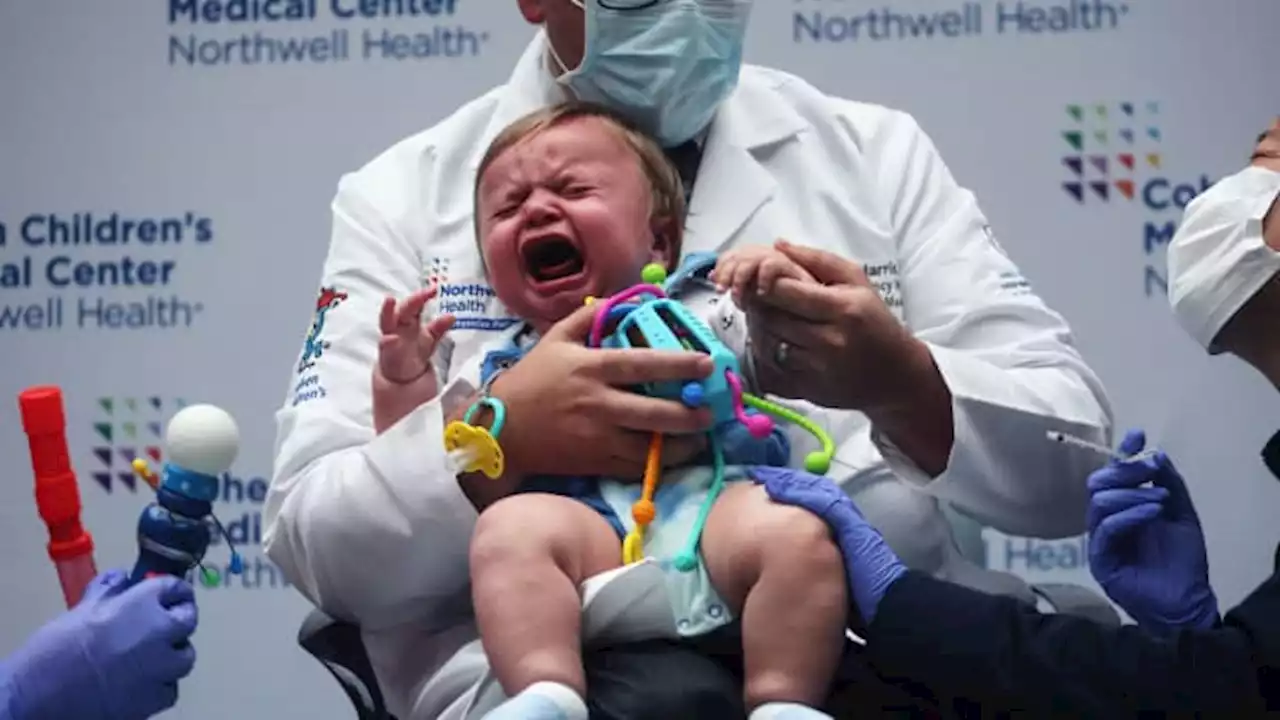 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Consulte Mais informação »
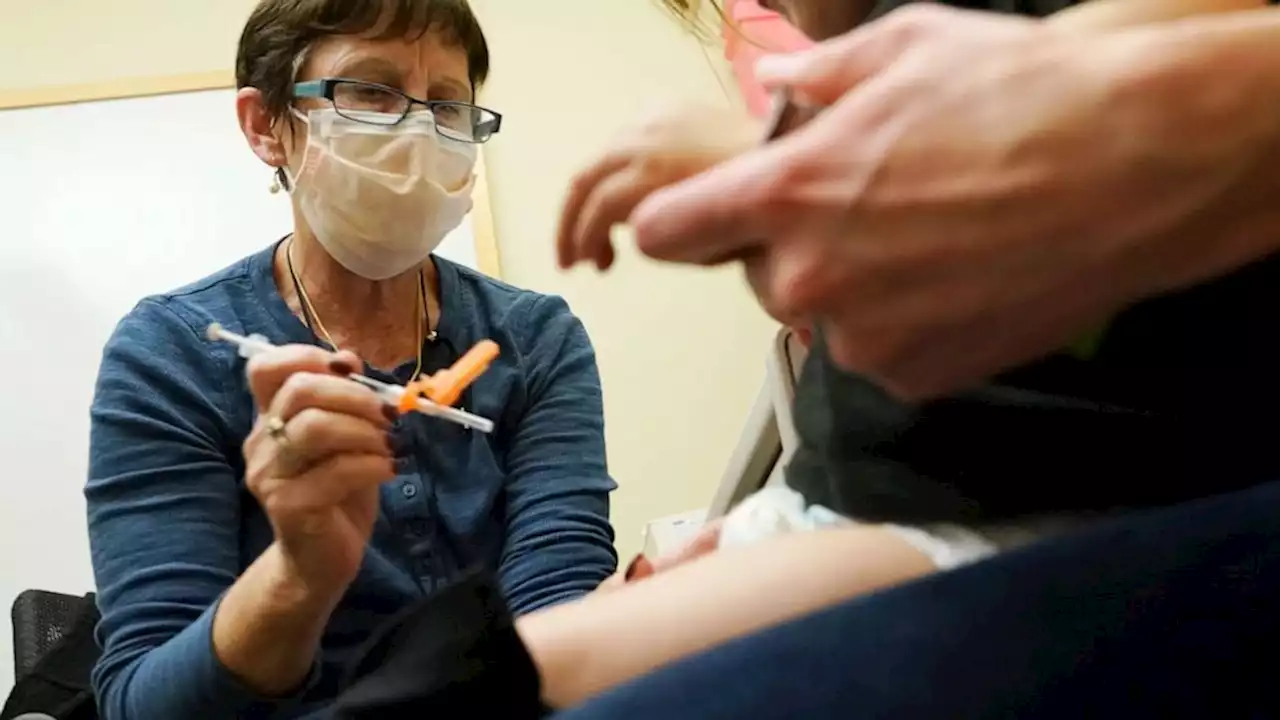 FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
Consulte Mais informação »
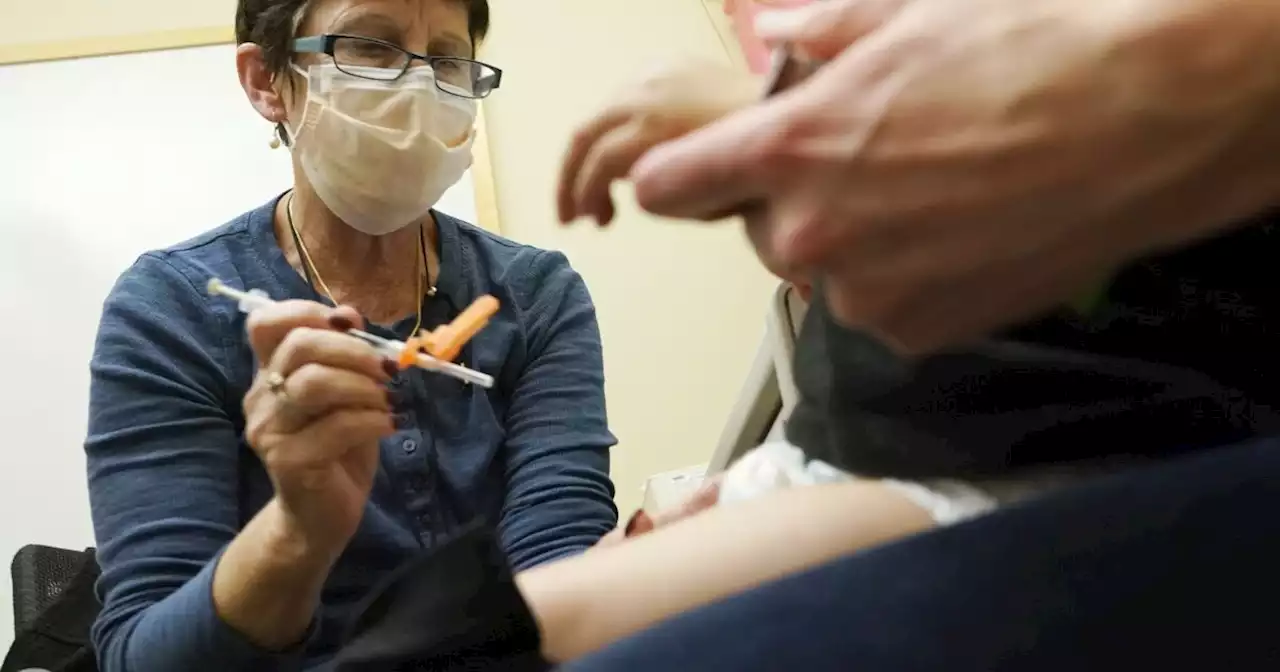 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
Consulte Mais informação »
