The FDA this morning authorized the first COVID-19 vaccines for children under 5, bringing shots for America's youngest children one step closer to reality.
for children under five, bringing shots for America's youngest children one step closer to a reality.authorized the Pfizer-BioNTech and Moderna COVID-19 vaccines
Following the FDA's authorization, a panel of independent advisers to the CDC will meet Saturday to discuss whether or not to recommend the shots "Many parents, caregivers and clinicians have been waiting for a vaccine for younger children and this action will help protect those down to 6 months of age," FDA Commissioner Robert Califf said in a statement. "As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of COVID-19, such as hospitalization and death.
“This is a long-awaited vaccine,” Dr. Jay Portnoy, a member of the panel who works at Children’s Hospital in Kansas City, Missouri, said at Wednesday's meeting. “There are so many parents who are absolutely desperate to get this vaccine and I think we owe it to them to give them a choice to have the vaccine if they want to.”
Dr. Walensky said at a Senate hearing that her staff will be working through the weekend – a federal holiday weekend commemorating Juneteenth – "because we understand the urgency of this for American parents." Moderna’s shots are one-quarter the dose of the company’s adult shots. Two doses appeared strong enough to prevent severe illness but only about 40% to 50% effective at preventing milder infections. Moderna has added a booster to its study and expects to eventually offer one.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Consulte Mais informação »
 FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
Consulte Mais informação »
 FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
Consulte Mais informação »
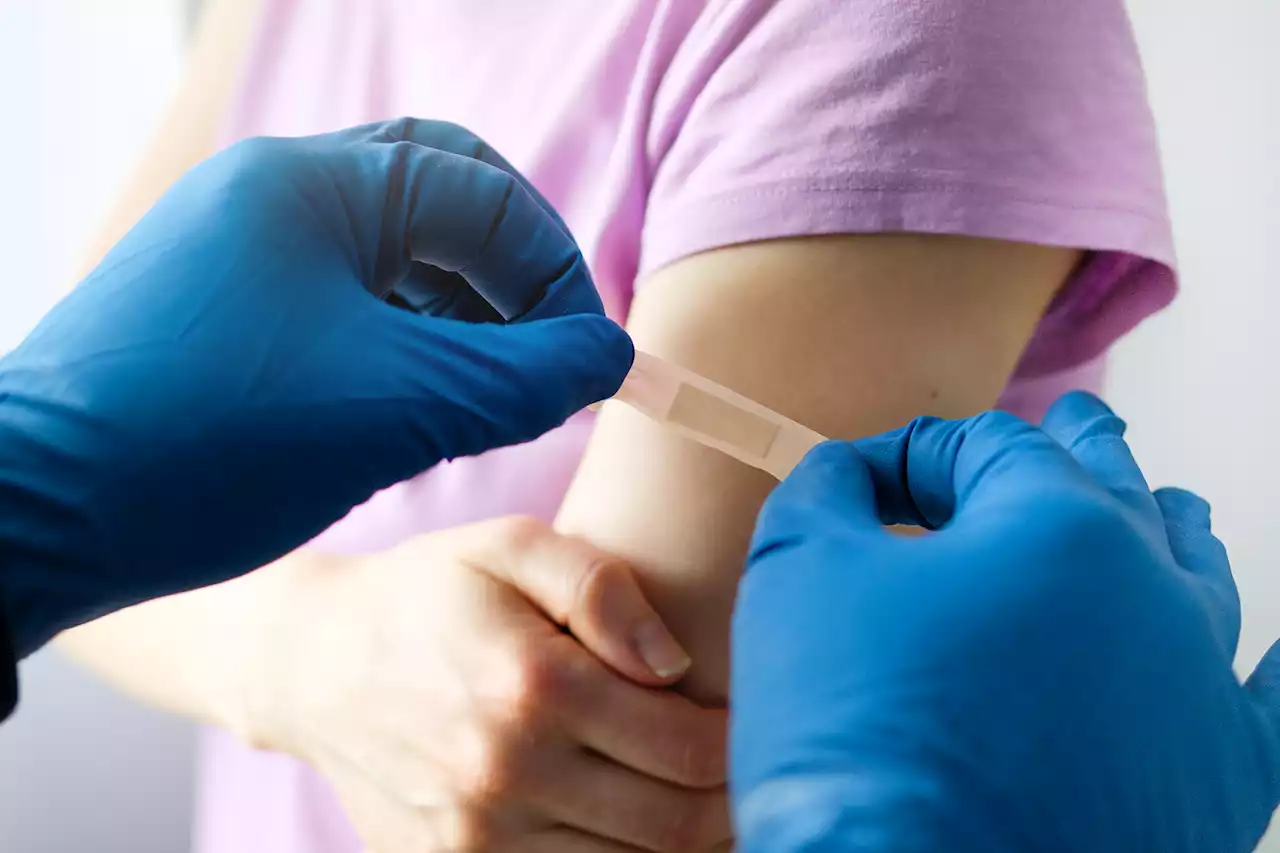 FDA Panel Recommends Moderna and Pfizer COVID-19 Vaccines for Children 6 Months and OlderIf the CDC also recommends the shots, everyone six months and older will be able to get a COVID-19 vaccine
FDA Panel Recommends Moderna and Pfizer COVID-19 Vaccines for Children 6 Months and OlderIf the CDC also recommends the shots, everyone six months and older will be able to get a COVID-19 vaccine
Consulte Mais informação »
 FDA panel greenlights COVID-19 vaccine for kids under 5COVID-19 shots for U.S. infants, toddlers and preschoolers moved a step closer Wednesday.
FDA panel greenlights COVID-19 vaccine for kids under 5COVID-19 shots for U.S. infants, toddlers and preschoolers moved a step closer Wednesday.
Consulte Mais informação »
