FDA authorizes use of Moderna and Pfizer's Covid-19 vaccines for children as young as 6 months.
The Moderna and Pfizer/BioNTech Covid-19 vaccines are now authorized for emergency use in young children. The US Food and Drug Administration expanded the authorizations for the vaccines Friday to include children as young as 6 months.
However, shots can't be given until the US Centers for Disease Control and Prevention's vaccine advisers have voted on whether to recommend them -- a vote is scheduled for Saturday -- and CDC Director Dr. Rochelle Walensky has signed off on that recommendation. The White House has said vaccinations for younger children may begin next week.Moderna's vaccine is now authorized for use in children 6 months through 17 years and Pfizer/BioNTech's for children 6 months through 4 years.
"We are dealing with an issue where I think we have to be careful that we don't become numb to the number of pediatric deaths because of the overwhelming number of older deaths here. Every life is important," he said, adding that"vaccine-preventable deaths are ones we would like to try to do something about.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA authorizes Pfizer, Moderna COVID-19 vaccines for kids under 5The FDA this morning authorized the first COVID-19 vaccines for children under 5, bringing shots for America's youngest children one step closer to reality.
FDA authorizes Pfizer, Moderna COVID-19 vaccines for kids under 5The FDA this morning authorized the first COVID-19 vaccines for children under 5, bringing shots for America's youngest children one step closer to reality.
Consulte Mais informação »
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Consulte Mais informação »
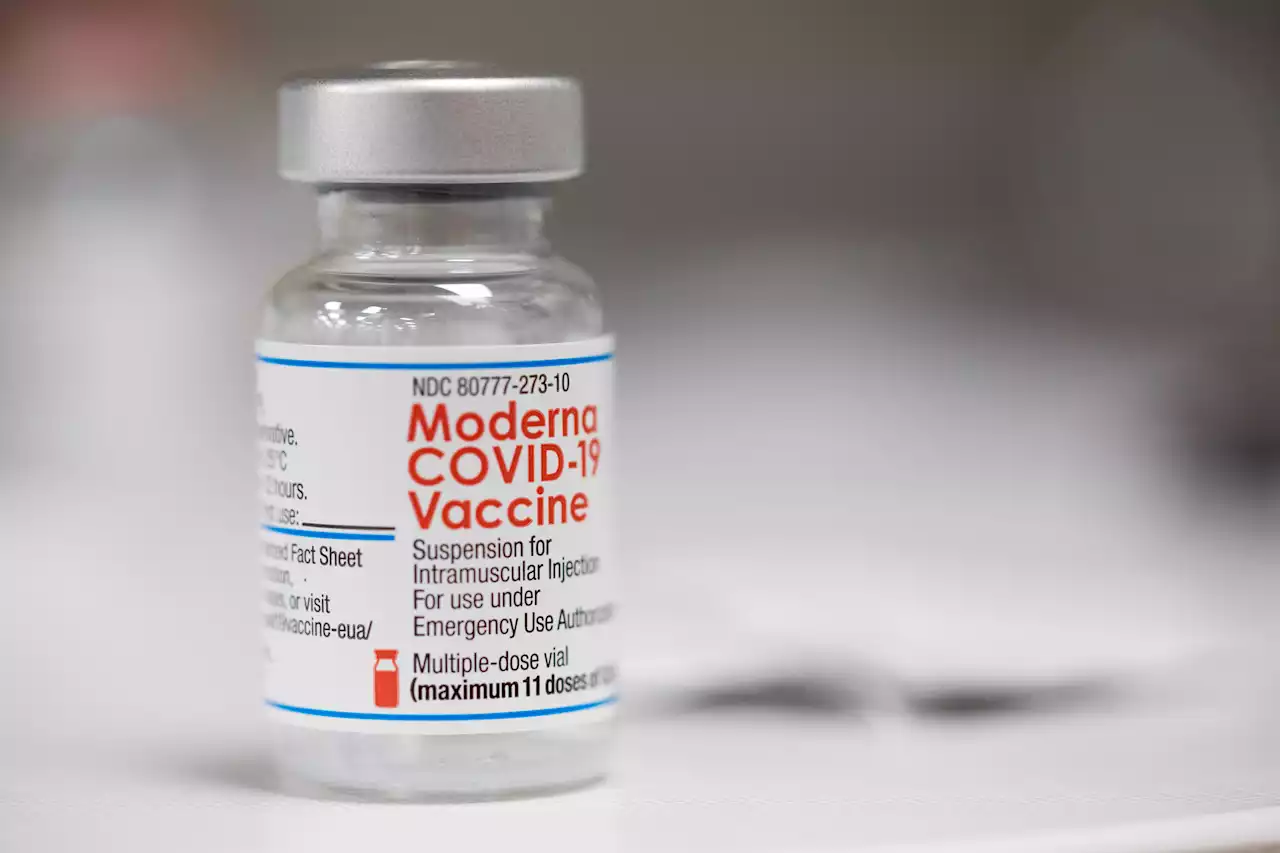 FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
Consulte Mais informação »
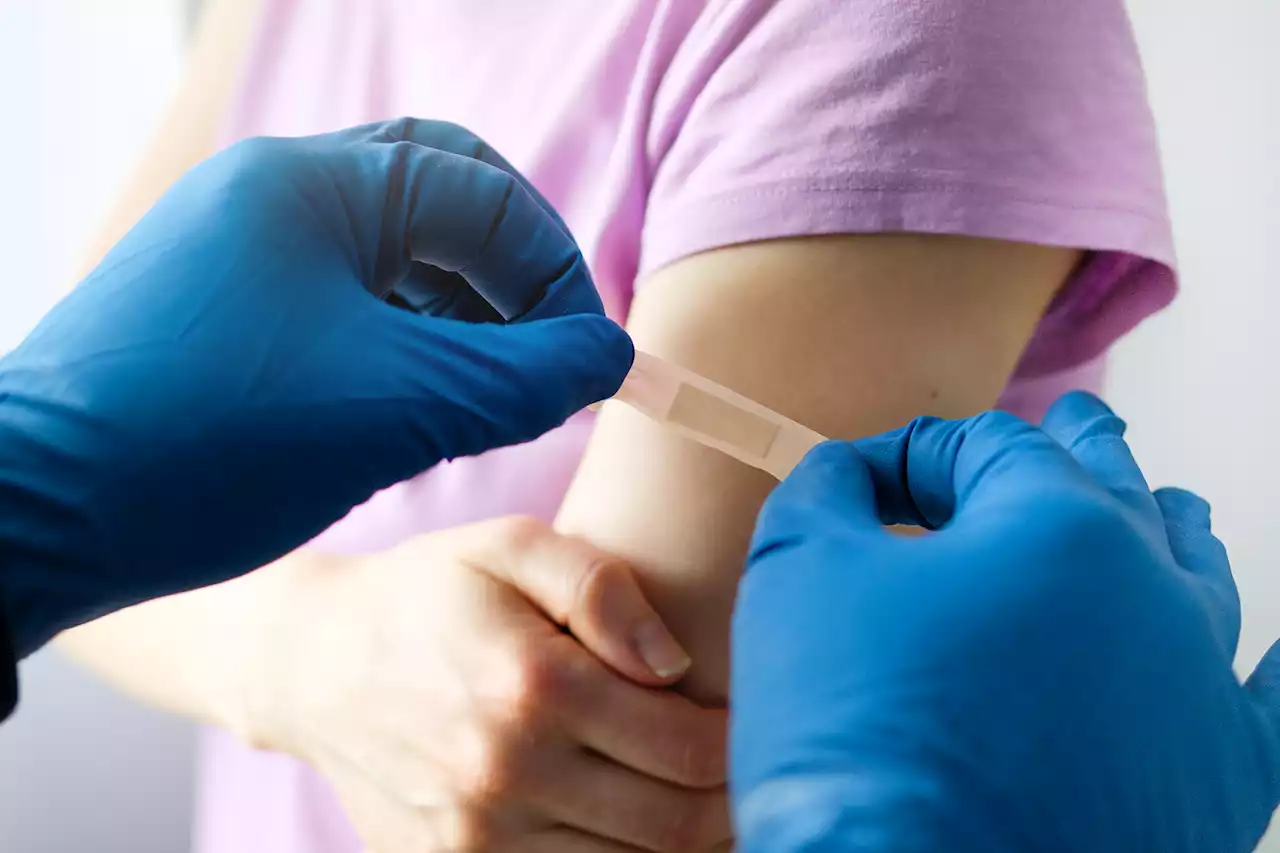 FDA Panel Recommends Moderna and Pfizer COVID-19 Vaccines for Children 6 Months and OlderIf the CDC also recommends the shots, everyone six months and older will be able to get a COVID-19 vaccine
FDA Panel Recommends Moderna and Pfizer COVID-19 Vaccines for Children 6 Months and OlderIf the CDC also recommends the shots, everyone six months and older will be able to get a COVID-19 vaccine
Consulte Mais informação »
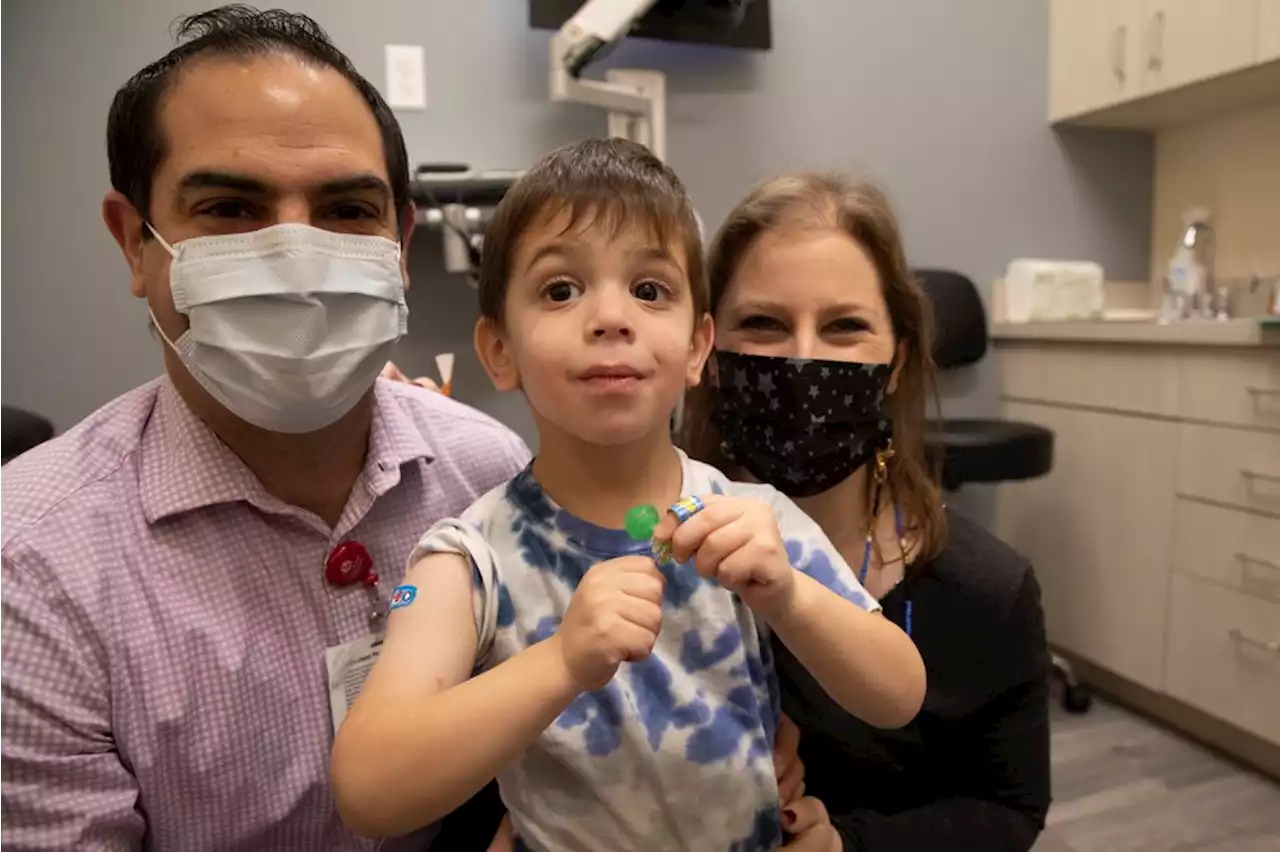 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Consulte Mais informação »
