Children ages 5 to 11 are now eligible to receive a COVID-19 booster, FDA says
White House COVID-19 coordinator Dr. Ashish Jha has urged everyone to get a bivalent booster shot by Oct. 31, ahead of the holidays and an expected winter surge in cases."Don't wait," Jha said in a White House briefing Tuesday."Get your new flu shot and get your new COVID shot today. If Americans did that, we could save hundreds of lives each day this winter."
The new shot is considered a"bivalent" vaccine, because it targets both the original virus and the variants that have been most common since early in the summer.seen in the United States, a figure that has fallen in recent weeks. BA.4.6 is now the second-most common variant after BA.5 and BA.7 is gaining ground, as well. It's not clear whether vaccines and treatments will respond any differently to those newer variants.
The Pfizer-BioNTech and Moderna boosters are considered equivalent, with no proven difference in terms of effectiveness or side effects for either one or for mixing vaccines. The Pfizer-BioNTech vaccine is available for children as young as 5, while Moderna's is only available for kids as young as 6.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA clears updated COVID boosters for kids as young as 5The U.S. on Wednesday authorized updated COVID-19 boosters for children as young as 5, seeking to expand protection ahead of an expected winter wave. Tweaked boosters rolled out for Americans 12 and older last month, doses modified to target today’s most common and contagious omicron relative .
FDA clears updated COVID boosters for kids as young as 5The U.S. on Wednesday authorized updated COVID-19 boosters for children as young as 5, seeking to expand protection ahead of an expected winter wave. Tweaked boosters rolled out for Americans 12 and older last month, doses modified to target today’s most common and contagious omicron relative .
Consulte Mais informação »
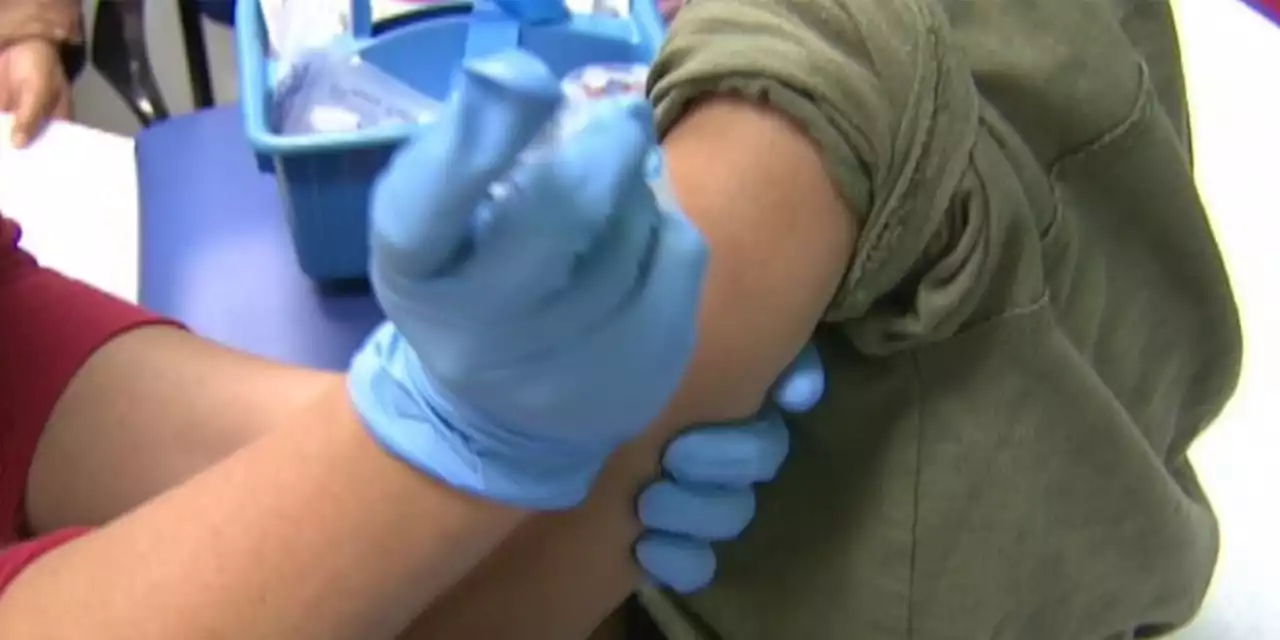 FDA clears updated COVID boosters for kids as young as 5Officials hope to expand protection against an expected winter surge.
FDA clears updated COVID boosters for kids as young as 5Officials hope to expand protection against an expected winter surge.
Consulte Mais informação »
 FDA clears updated COVID boosters for kids as young as 5The U.S. is about to offer updated COVID-19 booster shots to kids as young as 5
FDA clears updated COVID boosters for kids as young as 5The U.S. is about to offer updated COVID-19 booster shots to kids as young as 5
Consulte Mais informação »
 There's a spike in respiratory illness among children — and it's not just COVIDSick kids are crowding emergency rooms in parts of the country and some pediatric hospitals say they're running out of beds.
There's a spike in respiratory illness among children — and it's not just COVIDSick kids are crowding emergency rooms in parts of the country and some pediatric hospitals say they're running out of beds.
Consulte Mais informação »
