U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
The Food and Drug Administration’s action follows its advisory panel’s unanimous recommendation for the shots from Moderna and Pfizer. That means U.S. kids under 5 — roughly 18 million youngsters — are eligible for the shots, about 1 1/2 years after the vaccines first became available in the U.S. for adults, who have been hit the hardest during the pandemic.The FDA also authorized Moderna’s vaccine for school-aged children and teens.
She said pediatric deaths from COVID-19 have been higher than what is generally seen from the flu each year. For weeks, the Biden administration has been preparing to roll out the vaccines. States, tribes, community health centers and pharmacies preordered millions of doses. FDA’s emergency use authorization allows manufacturers to begin shipping vaccine across the country. Vaccinations could begin as early as Monday or Tuesday.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Consulte Mais informação »
 FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
Consulte Mais informação »
 FDA Authorizes 1st COVID-19 Shots for Infants, PreschoolersJUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
FDA Authorizes 1st COVID-19 Shots for Infants, PreschoolersJUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
Consulte Mais informação »
 FDA advisers endorse 1st COVID-19 shots for kids under 5The first COVID-19 shots for infants, toddlers and preschoolers in the U.S. have moved a step closer
FDA advisers endorse 1st COVID-19 shots for kids under 5The first COVID-19 shots for infants, toddlers and preschoolers in the U.S. have moved a step closer
Consulte Mais informação »
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Consulte Mais informação »
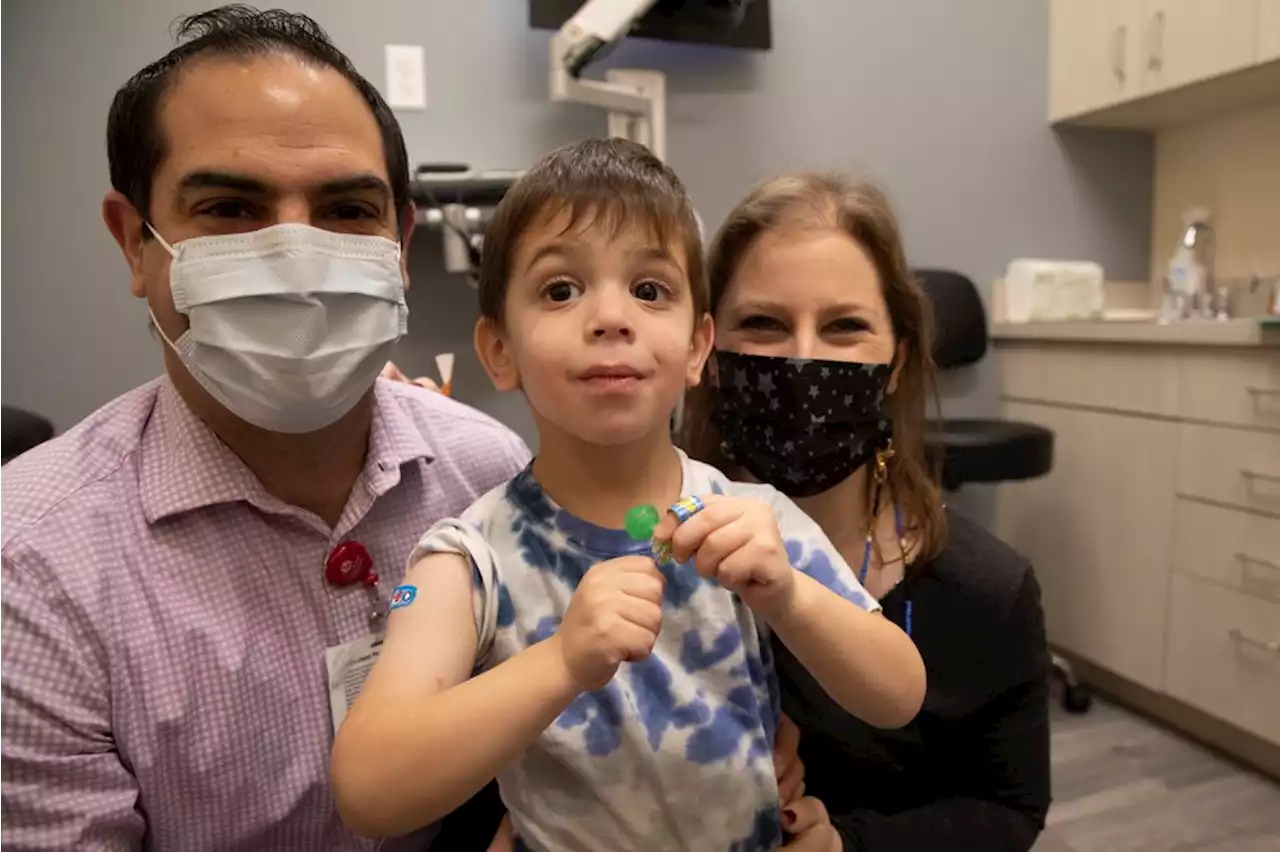 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Consulte Mais informação »