FDA authorizes 1st COVID-19 shots for infants, preschoolers.
While young children generally don’t get as sick from COVID-19 as older kids and adults, their hospitalizations surged during the omicron wave and FDA’s advisers determined that benefits from vaccination outweighed the minimal risks. Studies from Moderna and Pfizer showed side effects, including fever and fatigue, were mostly minor.Pfizer’s vaccine for kids younger than 5 is one-tenth of the adult dose.
Moderna’s is two shots, each a quarter of its adult dose, given about four weeks apart for kids younger than 6. The vaccines are for children as young as 6 months. Moderna next plans to study its shots for babies as young as 3 months old. Pfizer has not finalized plans for shots in younger infants. A dozen countries, including China, already vaccinate kids under 5.
Dr. Beth Ebel, professor of pediatrics at University of Washington in Seattle, said the tot-sized vaccines would be especially welcomed by U.S. parents with children in daycare where outbreaks can sideline parents from jobs, adding to financial strain. “A lot of people are going to be happy and a lot of grandparents are going to be happy, too, because we’ve missed those babies who grew up when you weren’t able to see them,” Ebel said.Follow AP Medical Writer Lindsey Tanner at @LindseyTanner.The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Consulte Mais informação »
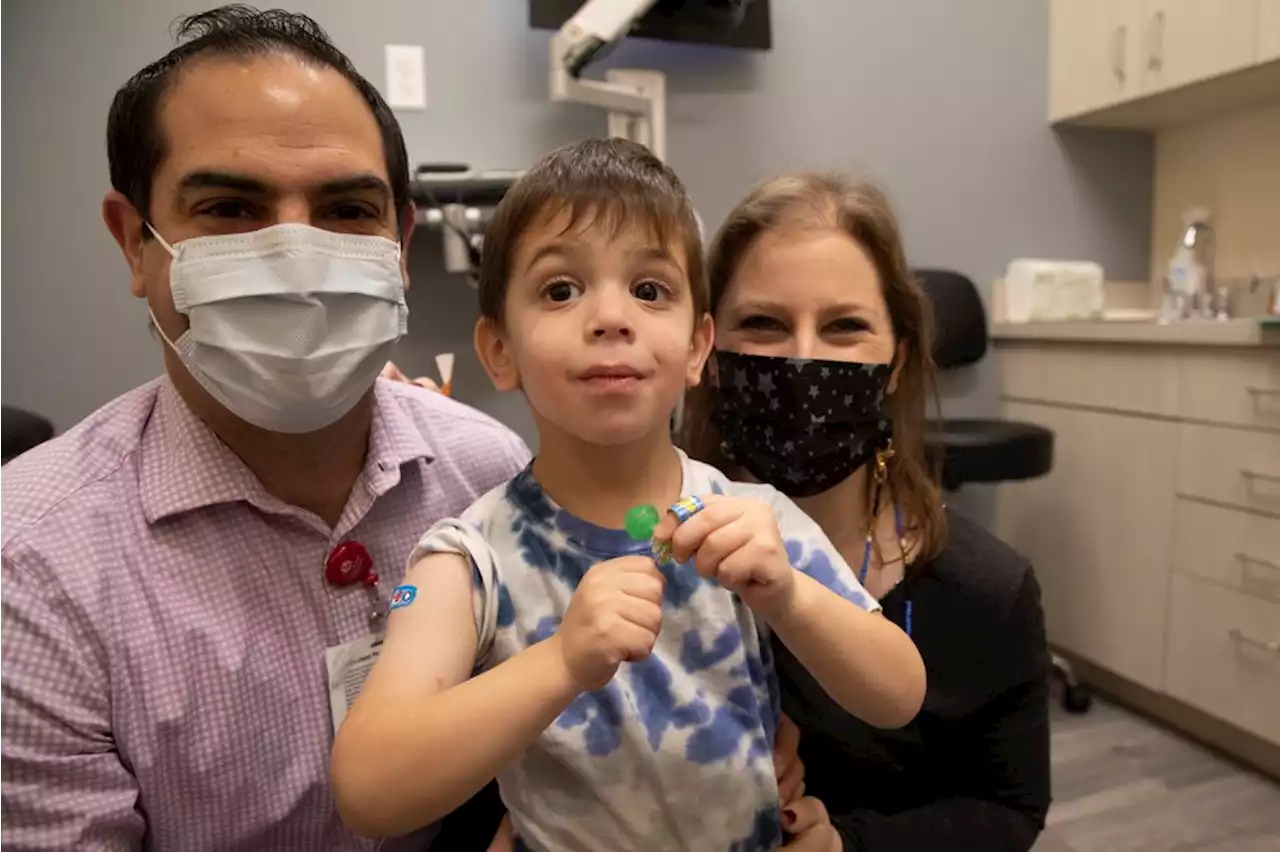 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Consulte Mais informação »
 FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
Consulte Mais informação »
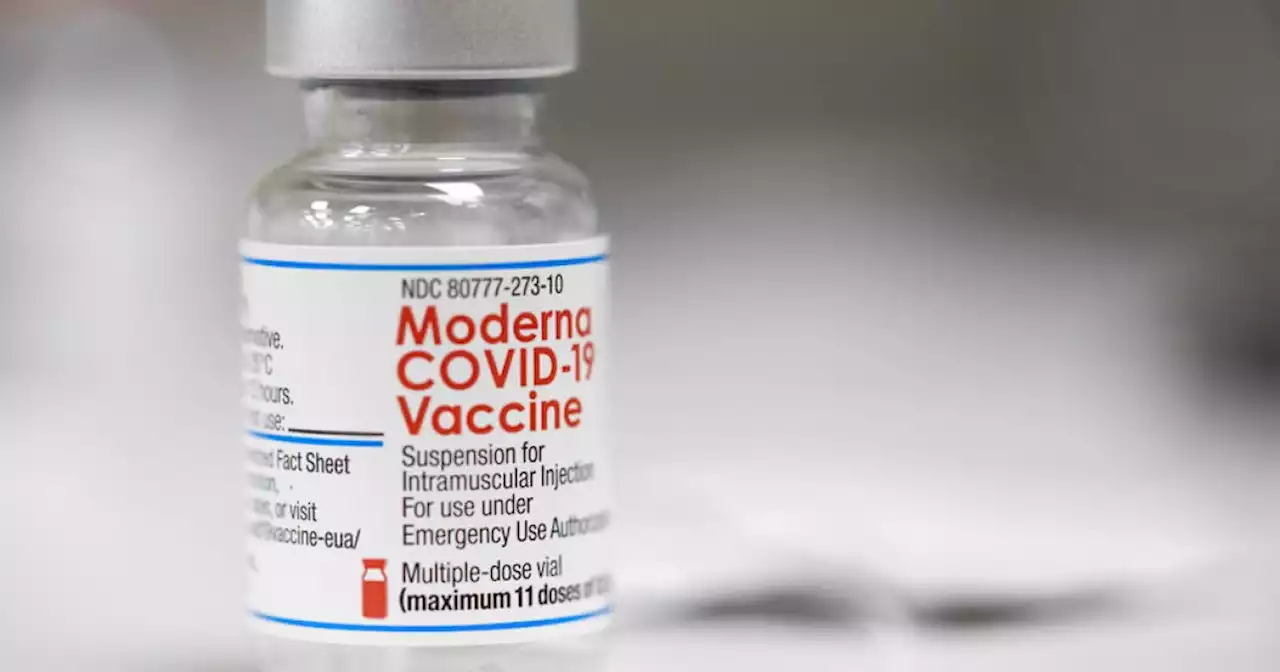 FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
Consulte Mais informação »
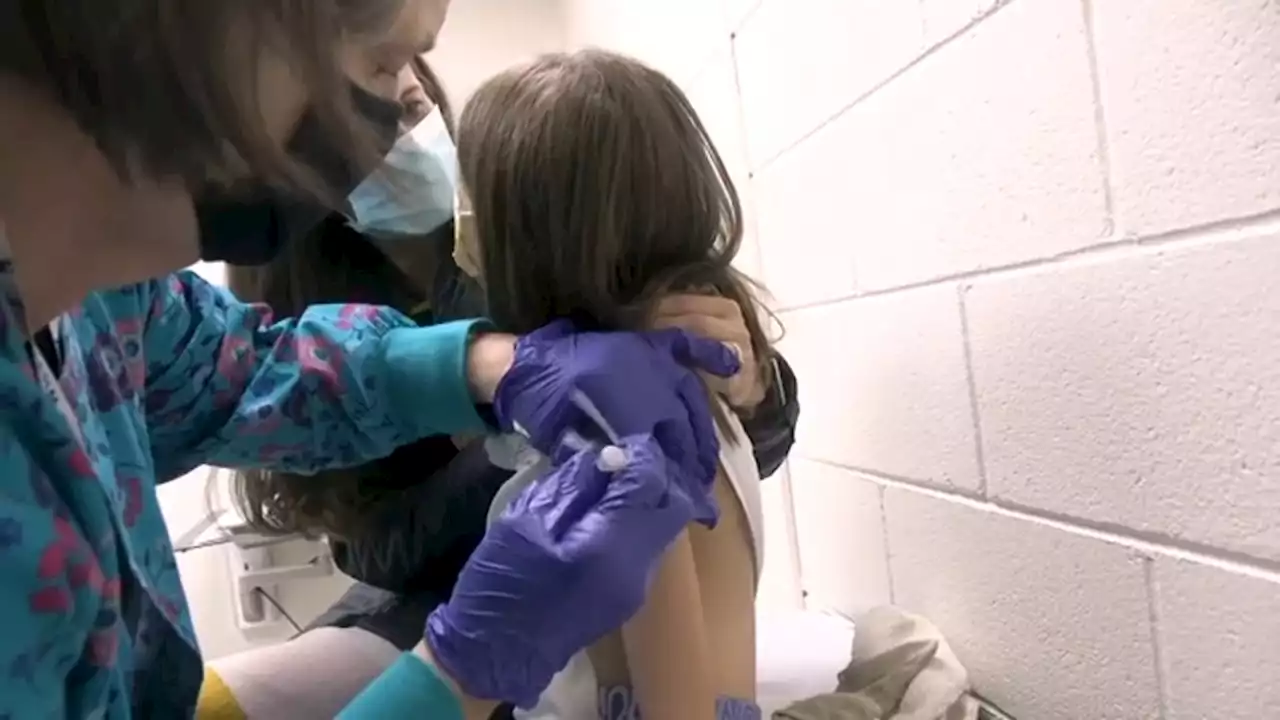 FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
Consulte Mais informação »
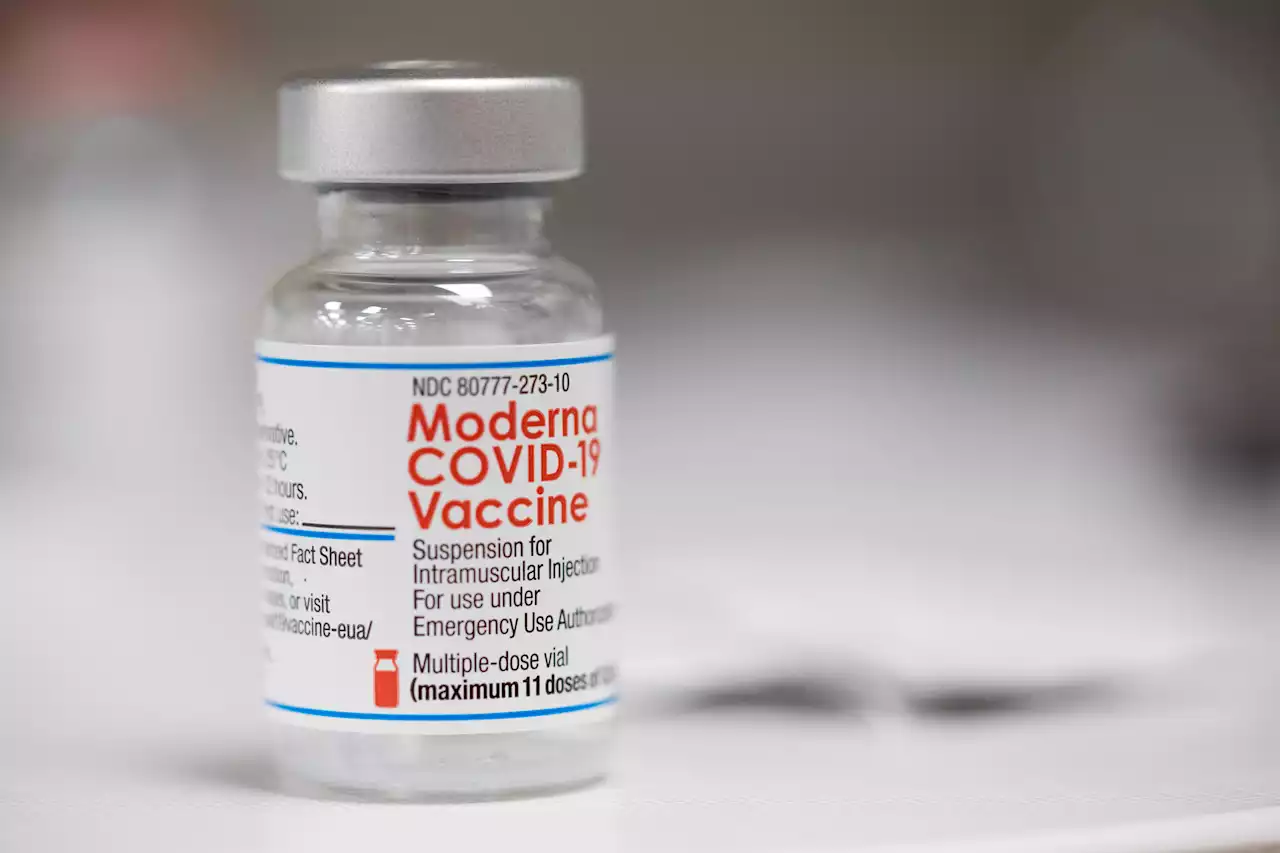 FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
Consulte Mais informação »
