FDAapproves JAK inhibitor upadacitinib for adults with moderately to severely active Crohns disease whose condition failed to respond adequately or who can't tolerate one or more TNF inhibitors.
The approval in Crohn's disease was supported by data from two induction studies and one maintenance study .
In the two induction studies, 857 patients were randomly assigned to receive upadacitinib 45 mg or placebo once daily for 12 weeks.
As at 12 week, at week 52, a greater proportion of patients who were treated with upadacitinib 15 mg or 30 mg, as compared to those who received placebo, achieved clinical remission, as indicated on the CDAI, and demonstrated improvement in intestinal inflammation, as assessed by colonoscopy.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA panel to vote on the first RSV vaccine for infants, given to pregnant mothersAn independent advisory committee to the FDA will decide on Thursday whether to recommend an RSV vaccine for infants. If approved, the shot, which is administered to pregnant mothers, would be the first of its kind.
FDA panel to vote on the first RSV vaccine for infants, given to pregnant mothersAn independent advisory committee to the FDA will decide on Thursday whether to recommend an RSV vaccine for infants. If approved, the shot, which is administered to pregnant mothers, would be the first of its kind.
Consulte Mais informação »
 FDA staff flag 'serious' safety risks for Intercept's fatty-liver disease treatmentThe U.S. health regulator's staff reviewers on Wednesday raised a string of concerns with Intercept Pharmaceuticals' treatment for a type of fatty liver disease, sending the drugmaker's shares plunging 22%.
FDA staff flag 'serious' safety risks for Intercept's fatty-liver disease treatmentThe U.S. health regulator's staff reviewers on Wednesday raised a string of concerns with Intercept Pharmaceuticals' treatment for a type of fatty liver disease, sending the drugmaker's shares plunging 22%.
Consulte Mais informação »
 Gerber infant formula continued being sold after recall, FDA saysThe formula continued to be sold in eight states despite the Food and Drug Administration issuing a recall in March.
Gerber infant formula continued being sold after recall, FDA saysThe formula continued to be sold in eight states despite the Food and Drug Administration issuing a recall in March.
Consulte Mais informação »
 FDA panel to vote on the first RSV vaccine for infants, administered to pregnant mothersIf eventually approved, the shot, which is made by Pfizer and administered to pregnant mothers, would be the first RSV vaccine for infants in the U.S.
FDA panel to vote on the first RSV vaccine for infants, administered to pregnant mothersIf eventually approved, the shot, which is made by Pfizer and administered to pregnant mothers, would be the first RSV vaccine for infants in the U.S.
Consulte Mais informação »
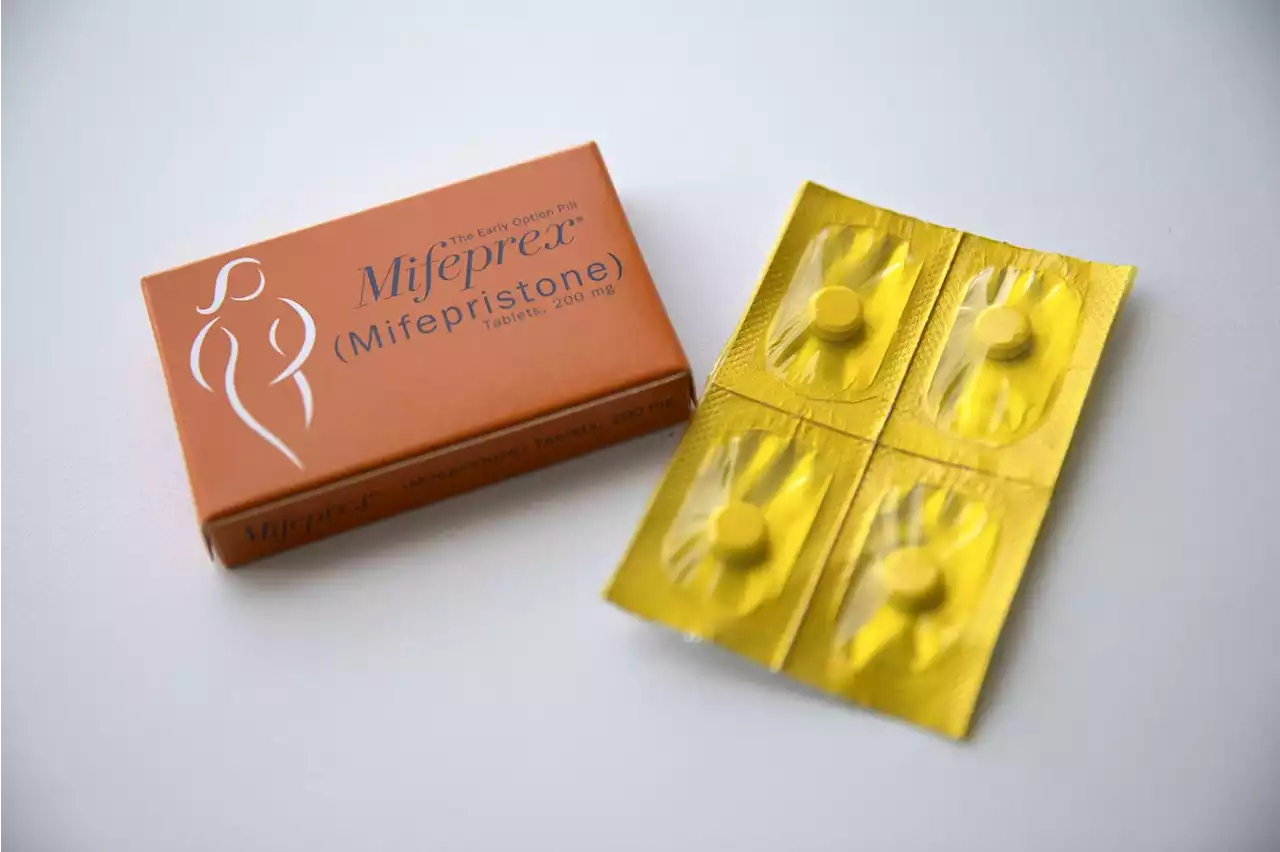 Abortion Pill Case Moves to Appeals Court, on Track for Supreme CourtAt issue are the FDA’s initial approval of mifepristone in 2000, and FDA actions making the drug more accessible in later years.
Abortion Pill Case Moves to Appeals Court, on Track for Supreme CourtAt issue are the FDA’s initial approval of mifepristone in 2000, and FDA actions making the drug more accessible in later years.
Consulte Mais informação »
 Appellate judges openly hostile to FDA and abortion pill maker at mifepristone hearingThe future of access to mifepristone, the most widely used abortion drug, looked doubtful after appellate judges in New Orleans defended the honor of an...
Appellate judges openly hostile to FDA and abortion pill maker at mifepristone hearingThe future of access to mifepristone, the most widely used abortion drug, looked doubtful after appellate judges in New Orleans defended the honor of an...
Consulte Mais informação »
