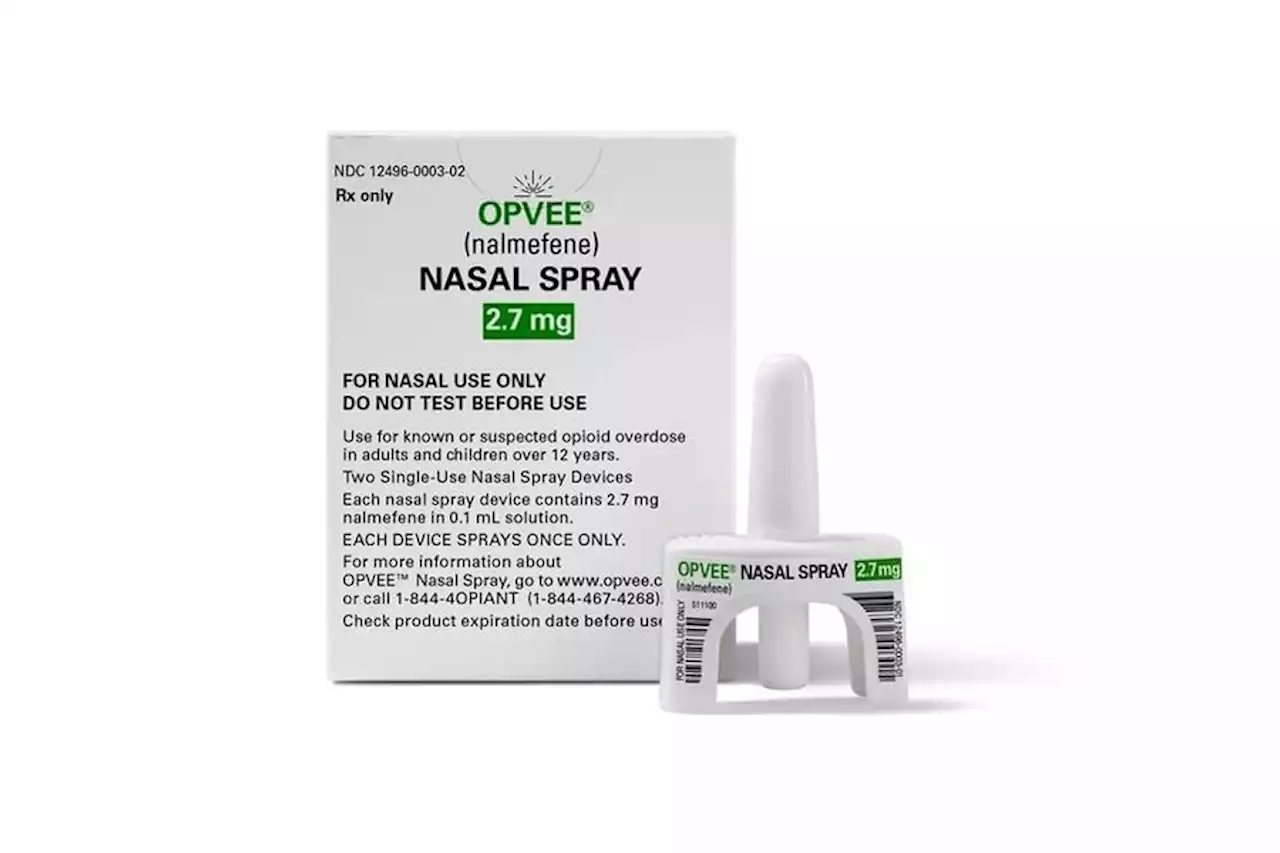
The FDA has approved a second nasal spray for reversing an opioid overdose.
TUESDAY, May 23, 2023 -- The U.S. Food and Drug Administration on Monday approved a second nasal spray for reversing an opioid overdose.
"On the heels of the FDA's recent approval of the first over-the-counter opioid reversal agent [Narcan], the availability of nalmefene nasal spray places a new prescription opioid reversal option in the hands of communities, harm reduction groups and emergency responders," Califf added. “Opvee is an emergency treatment for the fast reversal of respiratory depression triggered by natural or synthetic opioids, including fentanyl, and we are committed to making this novel rescue medication widely available to those who need it most to help save lives,” Crossley added.
That is why some health care providers prefer the shorter-lasting naloxone, with withdrawal symptoms that last 30 to 40 minutes, even if it needs to be given more than once.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA Approves New Indication for AvapritinibAvapritinib (AYVAKIT) is now approved to treat adults with indolent systemic mastocytosis.
FDA Approves New Indication for AvapritinibAvapritinib (AYVAKIT) is now approved to treat adults with indolent systemic mastocytosis.
Consulte Mais informação »
 FDA approves new drug to reverse opioid overdoseA new nasal spray can reverse the effects of an opioid overdose. The Food and Drug Administration gave its stamp of approval to Opvee, which can be used on patients 12 years and old...
FDA approves new drug to reverse opioid overdoseA new nasal spray can reverse the effects of an opioid overdose. The Food and Drug Administration gave its stamp of approval to Opvee, which can be used on patients 12 years and old...
Consulte Mais informação »
 FDA approves nasal spray to reverse fentanyl, other opioid overdosesU.S. health regulators on Monday approved a new easy-to-use version of a medication to reverse overdoses caused by fentanyl and other opioids driving the nation's drug crisis.
FDA approves nasal spray to reverse fentanyl, other opioid overdosesU.S. health regulators on Monday approved a new easy-to-use version of a medication to reverse overdoses caused by fentanyl and other opioids driving the nation's drug crisis.
Consulte Mais informação »
 FDA Approves Autoinjector Pen for Humira Biosimilar, CyltezoThe FDA approved a new autoinjection option for adalimumab-adbm, a biosimilar to AbbVie's adalimumab, ahead of Cyltezo's commercial launch on July 1, 2023.
FDA Approves Autoinjector Pen for Humira Biosimilar, CyltezoThe FDA approved a new autoinjection option for adalimumab-adbm, a biosimilar to AbbVie's adalimumab, ahead of Cyltezo's commercial launch on July 1, 2023.
Consulte Mais informação »
 FDA Approves First Pill to Treat Moderate-to-Severe Crohn's DiseaseRinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
FDA Approves First Pill to Treat Moderate-to-Severe Crohn's DiseaseRinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
Consulte Mais informação »
 New nasal spray to reverse fentanyl and other opioid overdoses gets FDA approvalOpvee is similar to naloxone, the life-saving drug that has been used for decades to quickly counter overdoses of heroin, fentanyl and prescription painkillers.
New nasal spray to reverse fentanyl and other opioid overdoses gets FDA approvalOpvee is similar to naloxone, the life-saving drug that has been used for decades to quickly counter overdoses of heroin, fentanyl and prescription painkillers.
Consulte Mais informação »