The FDA has approved extended-release injection buprenorphine for the treatment of moderate to severe opioid use disorder.
The US Food and Drug Administration has approved extended-release injection buprenorphine for the treatment of moderate to severe opioid use disorder .
"Buprenorphine is an important treatment option for opioid use disorder. Today's approval expands dosing options and provides people with opioid use disorder a greater opportunity to sustain long-term recovery," said FDA Commissioner Robert M. Califf, MD, in a release.
Brixadi is approved in both weekly and monthly subcutaneous injectable formulations at varying doses, including lower doses that may be appropriate for patients who do not tolerate higher doses of extended-release buprenorphine that are currently available.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA Approves First Pill to Treat Moderate-to-Severe Crohn's DiseaseRinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
FDA Approves First Pill to Treat Moderate-to-Severe Crohn's DiseaseRinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
Consulte Mais informação »
 FDA Approves Autoinjector Pen for Humira Biosimilar, CyltezoThe FDA approved a new autoinjection option for adalimumab-adbm, a biosimilar to AbbVie's adalimumab, ahead of Cyltezo's commercial launch on July 1, 2023.
FDA Approves Autoinjector Pen for Humira Biosimilar, CyltezoThe FDA approved a new autoinjection option for adalimumab-adbm, a biosimilar to AbbVie's adalimumab, ahead of Cyltezo's commercial launch on July 1, 2023.
Consulte Mais informação »
 FDA Approves New Indication for AvapritinibAvapritinib (AYVAKIT) is now approved to treat adults with indolent systemic mastocytosis.
FDA Approves New Indication for AvapritinibAvapritinib (AYVAKIT) is now approved to treat adults with indolent systemic mastocytosis.
Consulte Mais informação »
 FDA approves nasal spray to reverse fentanyl, other opioid overdosesU.S. health regulators on Monday approved a new easy-to-use version of a medication to reverse overdoses caused by fentanyl and other opioids driving the nation's drug crisis.
FDA approves nasal spray to reverse fentanyl, other opioid overdosesU.S. health regulators on Monday approved a new easy-to-use version of a medication to reverse overdoses caused by fentanyl and other opioids driving the nation's drug crisis.
Consulte Mais informação »
 FDA approves new drug to reverse opioid overdoseA new nasal spray can reverse the effects of an opioid overdose. The Food and Drug Administration gave its stamp of approval to Opvee, which can be used on patients 12 years and old...
FDA approves new drug to reverse opioid overdoseA new nasal spray can reverse the effects of an opioid overdose. The Food and Drug Administration gave its stamp of approval to Opvee, which can be used on patients 12 years and old...
Consulte Mais informação »
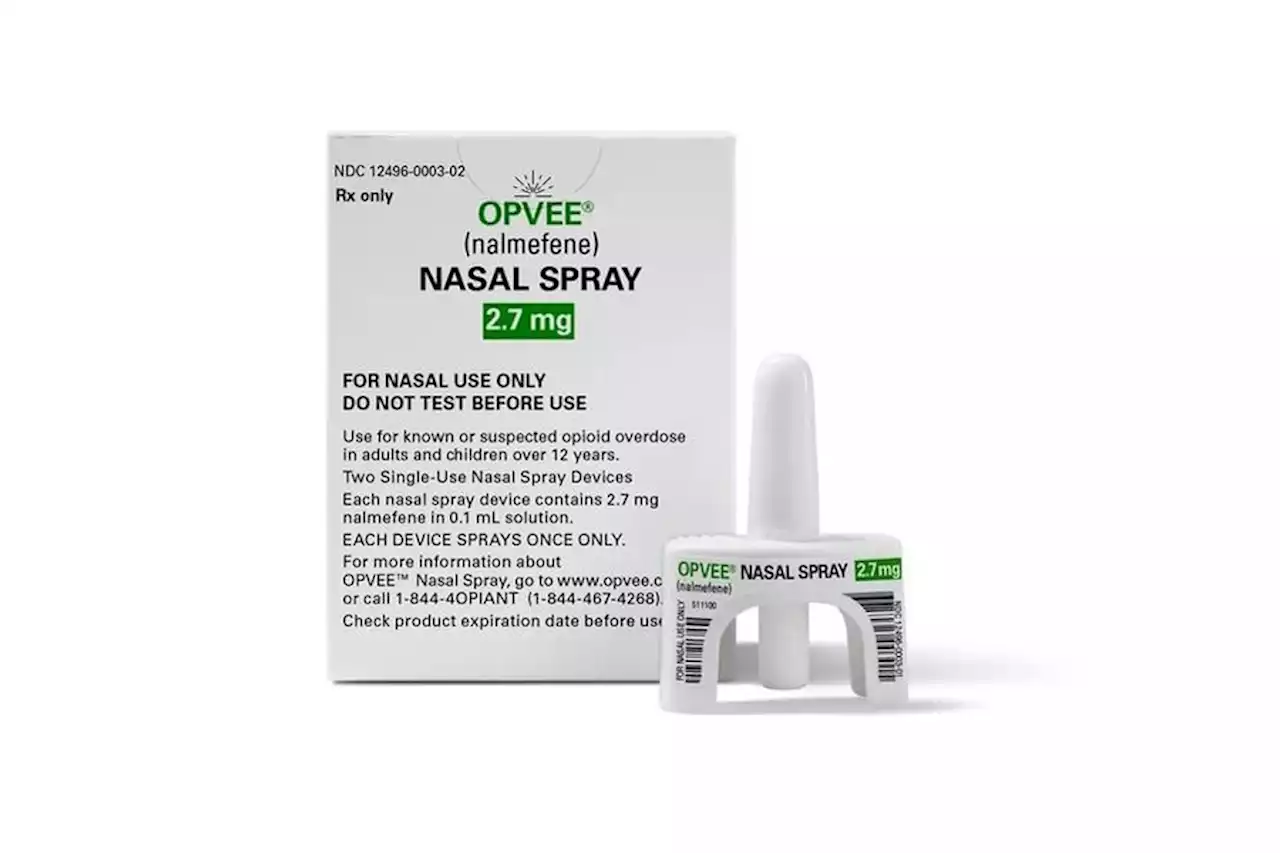 FDA Approves New Nasal Spray to Reverse OverdosesThe FDA has approved a second nasal spray for reversing an opioid overdose.
FDA Approves New Nasal Spray to Reverse OverdosesThe FDA has approved a second nasal spray for reversing an opioid overdose.
Consulte Mais informação »