U.S. drug regulators issued a report detailing quality control lapses at Novo Nordisk's main factory in North America as early as May last year, according to the report obtained by Reuters via a Freedom of Information Act request.
LONDON, Sept 20 - U.S. drug regulators issued a report detailing quality control lapses at Novo Nordisk's
There is no evidence that compliance failures flagged in the report known as a Form 483 resulted in harm to users of Wegovy and Ozempic. A Form 483 is a type of agency report containing "observations" that FDA inspectors "deem to be objectionable". One industry expert, who spoke on condition of anonymity due to the sensitivity of the matter, said similar issues at two inspections some time apart could expose the site to increased scrutiny from the FDA going forward.
The report included a second manufacturing lapse: "Failure to clean equipment at appropriate intervals" to prevent build-up of microorganisms on equipment. The report noted that the equipment in question was used for "continuous production" of batches of API.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 Oscars: Natalie Portman and Julianne Moore to Compete in Separate Categories for ‘May December’ (EXCLUSIVE)Natalie Portman and Julianne Moore are splitting up their Oscar campaigns for awards season. Although Todd Haynes’ delicious drama “May December” is interpreted by many as a two-h…
Oscars: Natalie Portman and Julianne Moore to Compete in Separate Categories for ‘May December’ (EXCLUSIVE)Natalie Portman and Julianne Moore are splitting up their Oscar campaigns for awards season. Although Todd Haynes’ delicious drama “May December” is interpreted by many as a two-h…
Consulte Mais informação »
 CDC, FDA Warn of Weight Loss Supplements That Might Contain a Hidden, Poisonous IngredientU.S. health agencies are flagging certain weight loss supplements that contain yellow oleander, a plant that can lead to severe gastrointestinal and cardiac issues.
CDC, FDA Warn of Weight Loss Supplements That Might Contain a Hidden, Poisonous IngredientU.S. health agencies are flagging certain weight loss supplements that contain yellow oleander, a plant that can lead to severe gastrointestinal and cardiac issues.
Consulte Mais informação »
 FDA advisers to discuss future of ‘artificial womb’ for human infantsIndependent advisers to the US Food and Drug Administration will meet Tuesday and Wednesday to discuss the regulations and ethics of creating an artificial womb to increase the chances that extremely premature babies would survive — and without long-term health problems.
FDA advisers to discuss future of ‘artificial womb’ for human infantsIndependent advisers to the US Food and Drug Administration will meet Tuesday and Wednesday to discuss the regulations and ethics of creating an artificial womb to increase the chances that extremely premature babies would survive — and without long-term health problems.
Consulte Mais informação »
 Natural Cycles gets FDA clearance to use Apple Watch temperature data for birth controlThe Apple Watch is the second wearable compatible with the digital contraceptive feature.
Natural Cycles gets FDA clearance to use Apple Watch temperature data for birth controlThe Apple Watch is the second wearable compatible with the digital contraceptive feature.
Consulte Mais informação »
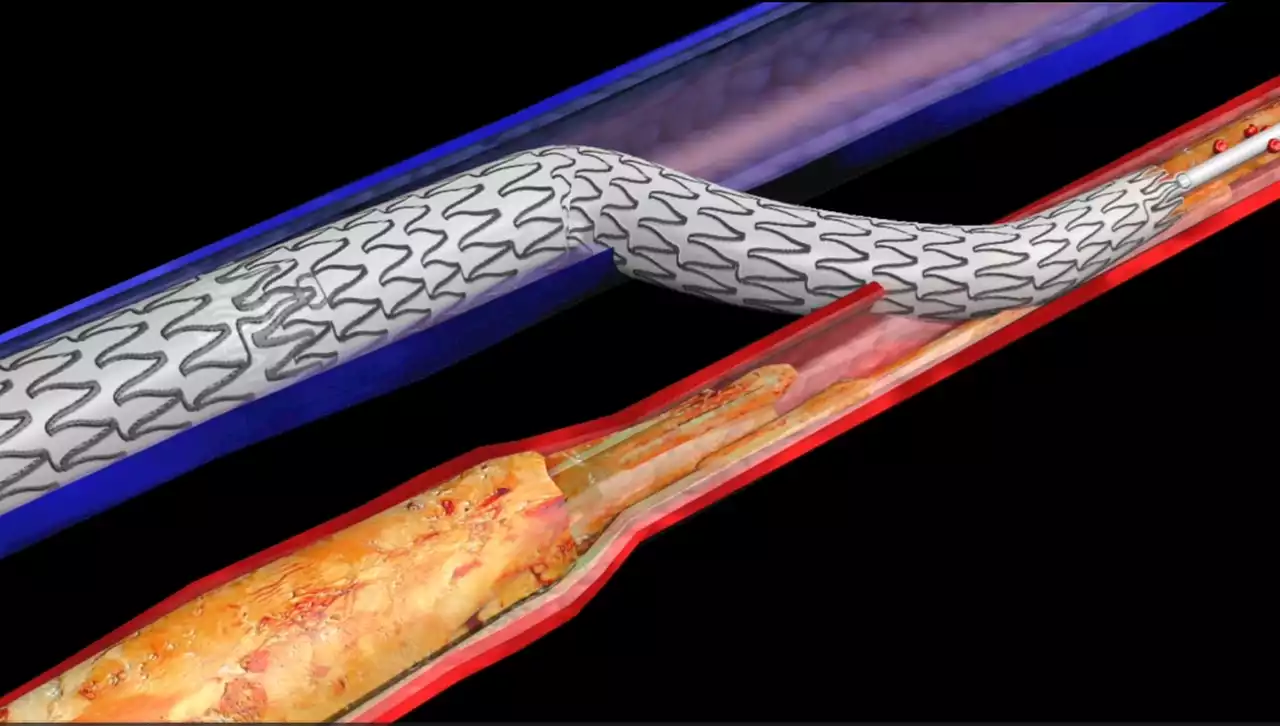 Technique to spare diabetic amputations pioneered at UH receives FDA approvalPioneered by researchers at University Hospitals Harrington Heart and Vascular Institute, LimFlow therapy bypasses blockages in the arteries of the legs that have been irreparably damaged by diabetes.
Technique to spare diabetic amputations pioneered at UH receives FDA approvalPioneered by researchers at University Hospitals Harrington Heart and Vascular Institute, LimFlow therapy bypasses blockages in the arteries of the legs that have been irreparably damaged by diabetes.
Consulte Mais informação »
FYI, The FDA Just Deemed A Bunch Of Cold & Flu Meds 'Ineffective'Everything to know about phenylephrine, the common cold and flu decongestant that apparently doesn't actually work.
Consulte Mais informação »
