Zepbound helped patients lose up to 48 pounds on average in clinical trials
The U.S. Food and Drug Administration on Wednesday approved Eli Lilly & Co.’s LLY, +1.74% obesity drug Zepbound, which in clinical trials helped patients lose up to 48 pounds on average.
Tirzepatide, the active ingredient in Zepbound, is already approved under the brand name Mounjaro for treatment of Type 2 diabetes. Zepbound is a once-weekly injection, similar to Novo Nordisk’s NVO, -0.44% Wegovy. In clinical trials, about one-third of patients taking the highest dose of Zepbound lost over 58 pounds, Lilly said. The average starting weight was 231 pounds, the company said.
The approval comes amid mounting evidence that the new generation of obesity drugs can also reduce cardiovascular risks. “In the long run, I see this as contributing to both better health and lower costs, which is an unusual opportunity for patients,” Dr. Daniel Skovronsky, chief scientific and medical officer at Eli Lilly, told MarketWatch in an interview in late October.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 FDA approves Eli Lilly’s Zepbound, a weight loss drug similar to Ozempic and WegovyBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
FDA approves Eli Lilly’s Zepbound, a weight loss drug similar to Ozempic and WegovyBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Consulte Mais informação »
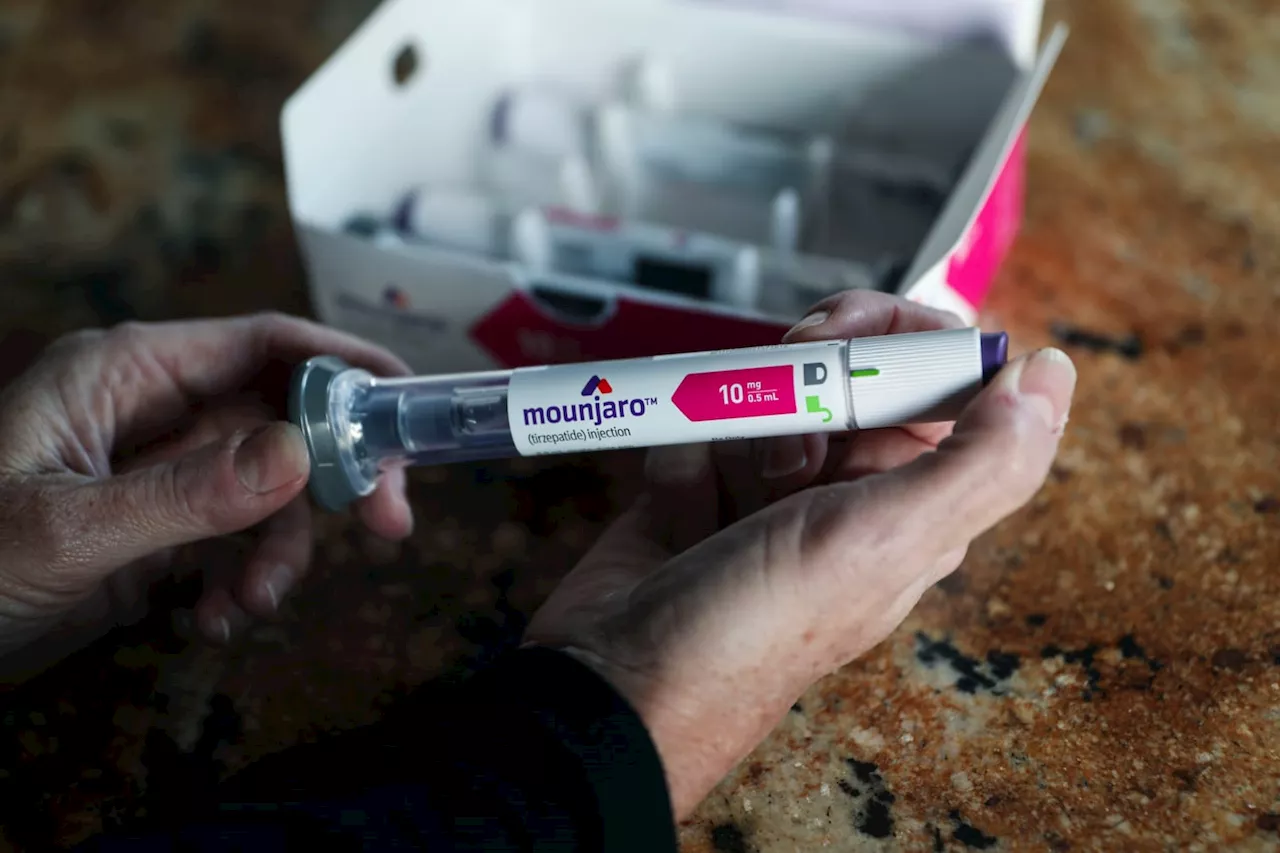 FDA approves new obesity drug from Eli Lilly named ZepboundThe decision by the Food and Drug Administration is expected to add to the demand for a new class of drugs transforming how patients battle obesity.
FDA approves new obesity drug from Eli Lilly named ZepboundThe decision by the Food and Drug Administration is expected to add to the demand for a new class of drugs transforming how patients battle obesity.
Consulte Mais informação »
 Eli Lilly's Obesity Drug Gets FDA ApprovalThe drug will be marketed as an obesity treatment under the name Zepbound.
Eli Lilly's Obesity Drug Gets FDA ApprovalThe drug will be marketed as an obesity treatment under the name Zepbound.
Consulte Mais informação »
 Zepbound: FDA approves new version of diabetes drug Mounjaro for weight lossThe U.S. Food and Drug Administration on Wednesday approved a new version of the type 2 diabetes drug tirzepatide for use in chronic weight management, officials said.
Zepbound: FDA approves new version of diabetes drug Mounjaro for weight lossThe U.S. Food and Drug Administration on Wednesday approved a new version of the type 2 diabetes drug tirzepatide for use in chronic weight management, officials said.
Consulte Mais informação »
 FDA approves diabetes drug Zepbound to help obese patients lose weightStudies suggest Zepbound can help people lose weight with diet and exercise.
FDA approves diabetes drug Zepbound to help obese patients lose weightStudies suggest Zepbound can help people lose weight with diet and exercise.
Consulte Mais informação »
 Eli Lilly’s Mounjaro Approved for Weight Loss in the U.S.The drug, which will be sold as Zepbound as an anti-obesity treatment, had previously been cleared by the FDA for the treatment of Type 2 diabetes
Eli Lilly’s Mounjaro Approved for Weight Loss in the U.S.The drug, which will be sold as Zepbound as an anti-obesity treatment, had previously been cleared by the FDA for the treatment of Type 2 diabetes
Consulte Mais informação »
