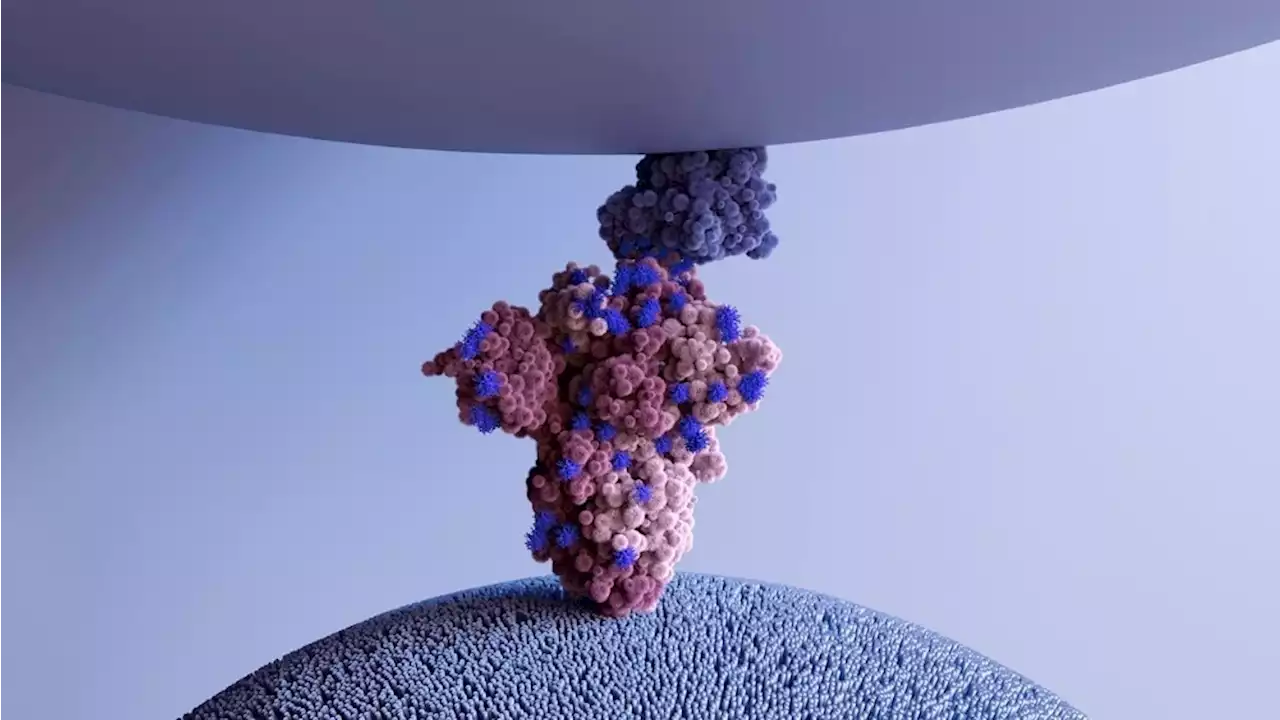
Decoy ACE2 receptors broadly neutralize SARS-CoV-2 variants ACE2 Coronavirus Disease COVID SARSCoV2 CellPressNews osaka_univ_e KyotoU_News
By Dr. Priyom Bose, Ph.D.Jul 31 2022Reviewed by Benedette Cuffari, M.Sc. Previously, researchers have developed decoy receptor proteins to combat the problem of viruses escaping the host immune response. The main idea behind this strategy is that viruses cannot resist binding to the decoy receptors instead of the host receptor during the invasion.
Thus, scientists are extremely interested in analyzing soluble ACE2 as a potential candidate for a decoy receptor against coronaviruses. In a recent Trends in Pharmacological Science journal study, researchers review current developments in preclinical studies associated with recombinant ACE2 decoy receptors against coronaviruses.
About the study In the current study, scientists assess the use of engineered ACE2 decoy as an alternative approach to combat problems related to SARS-CoV-2 genomic evolutions. These receptors can neutralize viruses in a manner that is extremely effective against virus escapability. Clinical studies related to the application of rhACE2 in both healthy volunteers and ARDS patients have shown no beneficial changes with respect to acute clinical outcomes. However, both study groups showed no adverse reaction to the drug.
The therapeutic potential of rhACE2 as a decoy receptor against COVID-19 could be improved through ACE2 mutagenesis approaches. This strategy can enhance binding affinity and fusion to Fc, which could prolong the half-life of these agents, as well as enhance antiviral immune responses and avidity effects, both of which are essential features of effective COVID-19 therapeutics.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 A systematic analysis of splicing variants identifies new diagnoses in the 100,000 Genomes Project - Genome MedicineBackground Genomic variants which disrupt splicing are a major cause of rare genetic diseases. However, variants which lie outside of the canonical splice sites are difficult to interpret clinically. Improving the clinical interpretation of non-canonical splicing variants offers a major opportunity to uplift diagnostic yields from whole genome sequencing data. Methods Here, we examine the landscape of splicing variants in whole-genome sequencing data from 38,688 individuals in the 100,000 Genomes Project and assess the contribution of non-canonical splicing variants to rare genetic diseases. We use a variant-level constraint metric (the mutability-adjusted proportion of singletons) to identify constrained functional variant classes near exon–intron junctions and at putative splicing branchpoints. To identify new diagnoses for individuals with unsolved rare diseases in the 100,000 Genomes Project, we identified individuals with de novo single-nucleotide variants near exon–intron boundaries and at putative splicing branchpoints in known disease genes. We identified candidate diagnostic variants through manual phenotype matching and confirmed new molecular diagnoses through clinical variant interpretation and functional RNA studies. Results We show that near-splice positions and splicing branchpoints are highly constrained by purifying selection and harbour potentially damaging non-coding variants which are amenable to systematic analysis in sequencing data. From 258 de novo splicing variants in known rare disease genes, we identify 35 new likely diagnoses in probands with an unsolved rare disease. To date, we have confirmed a new diagnosis for six individuals, including four in whom RNA studies were performed. Conclusions Overall, we demonstrate the clinical value of examining non-canonical splicing variants in individuals with unsolved rare diseases.
A systematic analysis of splicing variants identifies new diagnoses in the 100,000 Genomes Project - Genome MedicineBackground Genomic variants which disrupt splicing are a major cause of rare genetic diseases. However, variants which lie outside of the canonical splice sites are difficult to interpret clinically. Improving the clinical interpretation of non-canonical splicing variants offers a major opportunity to uplift diagnostic yields from whole genome sequencing data. Methods Here, we examine the landscape of splicing variants in whole-genome sequencing data from 38,688 individuals in the 100,000 Genomes Project and assess the contribution of non-canonical splicing variants to rare genetic diseases. We use a variant-level constraint metric (the mutability-adjusted proportion of singletons) to identify constrained functional variant classes near exon–intron junctions and at putative splicing branchpoints. To identify new diagnoses for individuals with unsolved rare diseases in the 100,000 Genomes Project, we identified individuals with de novo single-nucleotide variants near exon–intron boundaries and at putative splicing branchpoints in known disease genes. We identified candidate diagnostic variants through manual phenotype matching and confirmed new molecular diagnoses through clinical variant interpretation and functional RNA studies. Results We show that near-splice positions and splicing branchpoints are highly constrained by purifying selection and harbour potentially damaging non-coding variants which are amenable to systematic analysis in sequencing data. From 258 de novo splicing variants in known rare disease genes, we identify 35 new likely diagnoses in probands with an unsolved rare disease. To date, we have confirmed a new diagnosis for six individuals, including four in whom RNA studies were performed. Conclusions Overall, we demonstrate the clinical value of examining non-canonical splicing variants in individuals with unsolved rare diseases.
Consulte Mais informação »
 Covid infections could give just 28 days immunity as experts warn of shorter, sharper wavesScientists have warned people could face being infected with Covid several times a year after four peaks in the Omicron variant within seven months
Covid infections could give just 28 days immunity as experts warn of shorter, sharper wavesScientists have warned people could face being infected with Covid several times a year after four peaks in the Omicron variant within seven months
Consulte Mais informação »
 Covid 'holy grail' vaccine could target multiple variantsScientists at the Francis Crick Institute believe they found a genetic feature of the covid virus that appeared 'similar' to a number of coronaviruses that are less prone to mutations which could prove as a 'holy grail' vaccine.
Covid 'holy grail' vaccine could target multiple variantsScientists at the Francis Crick Institute believe they found a genetic feature of the covid virus that appeared 'similar' to a number of coronaviruses that are less prone to mutations which could prove as a 'holy grail' vaccine.
Consulte Mais informação »