The CDC's vaccine advisers are expected to vote Saturday to allow children under 5 to receive COVID-19 vaccines.
The move comes after the Food and Drug Administration authorized Moderna’s and Pfizer’s COVID-19 shots for that age group on Friday.
Pfizer would come as a three-dose vaccine for children 6 months to 4 years old, and Moderna’s two-dose vaccine would be for children 6 months to 5 years old.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Consulte Mais informação »
 More than one-third of Colorado counties at high COVID risk, CDC saysThe San Luis Valley and parts of the Eastern Plains and high country have joined the Denver area and the Western Slope at the high COVID-19 risk level, according to the Centers for Disease Control and Prevention.
More than one-third of Colorado counties at high COVID risk, CDC saysThe San Luis Valley and parts of the Eastern Plains and high country have joined the Denver area and the Western Slope at the high COVID-19 risk level, according to the Centers for Disease Control and Prevention.
Consulte Mais informação »
 CDC Advisers Reviewing Covid-19 Vaccines for Young ChildrenA recommendation from the advisers, who are meeting Friday and Saturday to review data around vaccinating those under 5 years old, could pave the way for expanding access to the shots.
CDC Advisers Reviewing Covid-19 Vaccines for Young ChildrenA recommendation from the advisers, who are meeting Friday and Saturday to review data around vaccinating those under 5 years old, could pave the way for expanding access to the shots.
Consulte Mais informação »
 FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Consulte Mais informação »
 FDA authorizes COVID vaccine for children under 5, CDC review nextAn FDA advisory panel unanimously found both vaccines to be “safe and effective” for children as young as 6 months old.
FDA authorizes COVID vaccine for children under 5, CDC review nextAn FDA advisory panel unanimously found both vaccines to be “safe and effective” for children as young as 6 months old.
Consulte Mais informação »
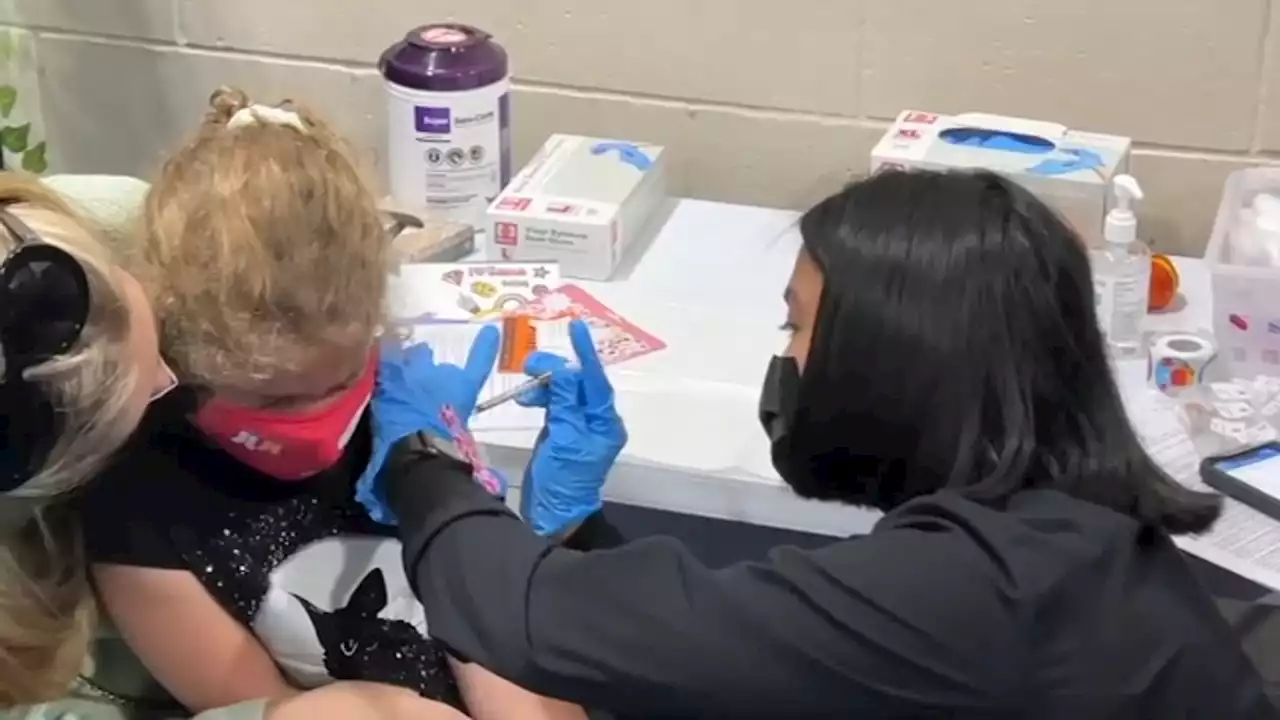 CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
Consulte Mais informação »
