CDC Director Rochelle Walensky gave her seal of approval on COVID-19 vaccines for everyone 6 months or older on Saturday, meaning children can immediately begin to get vaccinated
. The decision follows the CDC’s Advisory Committee on Immunization Practices unanimous vote to recommend vaccines for children older than six months. Parents have been waiting for nearly two and a half years for this authorization. “Vaccinating young children is a critical opportunity to protect them against hospitalization and death from COVID-19,” Walensky said in a video statement.
“I hope all parents will take advantage of these life-saving vaccines.” Health experts are urging parents to vaccinate their children. “We want to say today that if you’re not going to immunize your children, we think that’s a misplaced concern and that you should immunize your children to save their lives," committee member Dr. Sarah Long, a pediatrician at St. Christopher’s Hospital for Children in Philadelphia, told NBC.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
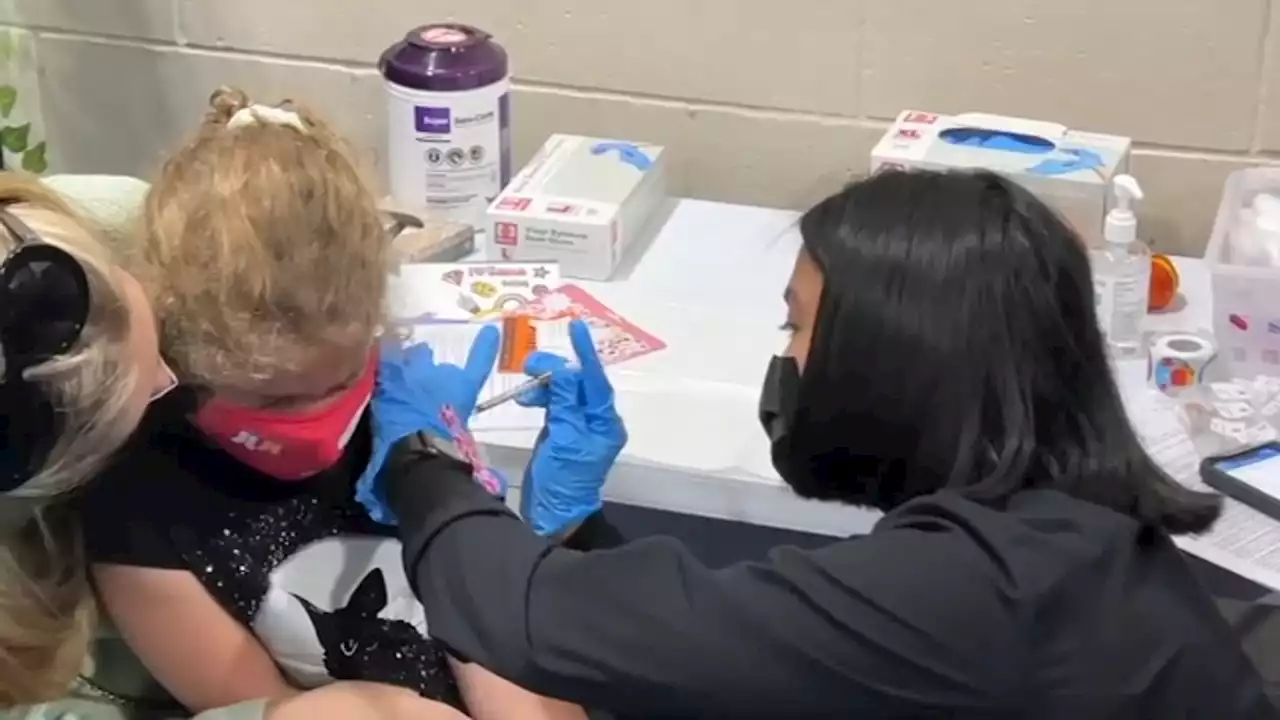 CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
Consulte Mais informação »
 CDC Advisers Reviewing Covid-19 Vaccines for Young ChildrenA recommendation from the advisers, who are meeting Friday and Saturday to review data around vaccinating those under 5 years old, could pave the way for expanding access to the shots.
CDC Advisers Reviewing Covid-19 Vaccines for Young ChildrenA recommendation from the advisers, who are meeting Friday and Saturday to review data around vaccinating those under 5 years old, could pave the way for expanding access to the shots.
Consulte Mais informação »
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Consulte Mais informação »
 FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Consulte Mais informação »
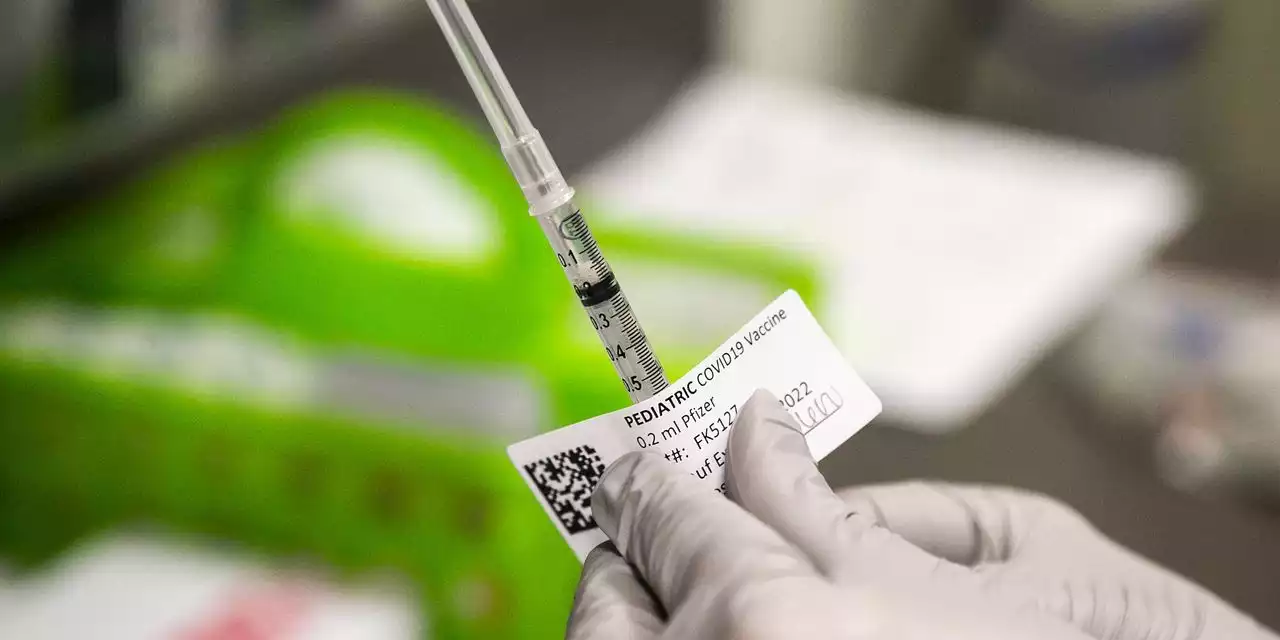 CDC Recommends Covid-19 Vaccines for Young ChildrenVaccine experts advising the Centers for Disease Control and Prevention voted to recommend Covid-19 shots for young children ages 6 months to 5 years, who could potentially start receiving shots as soon as Monday.
CDC Recommends Covid-19 Vaccines for Young ChildrenVaccine experts advising the Centers for Disease Control and Prevention voted to recommend Covid-19 shots for young children ages 6 months to 5 years, who could potentially start receiving shots as soon as Monday.
Consulte Mais informação »
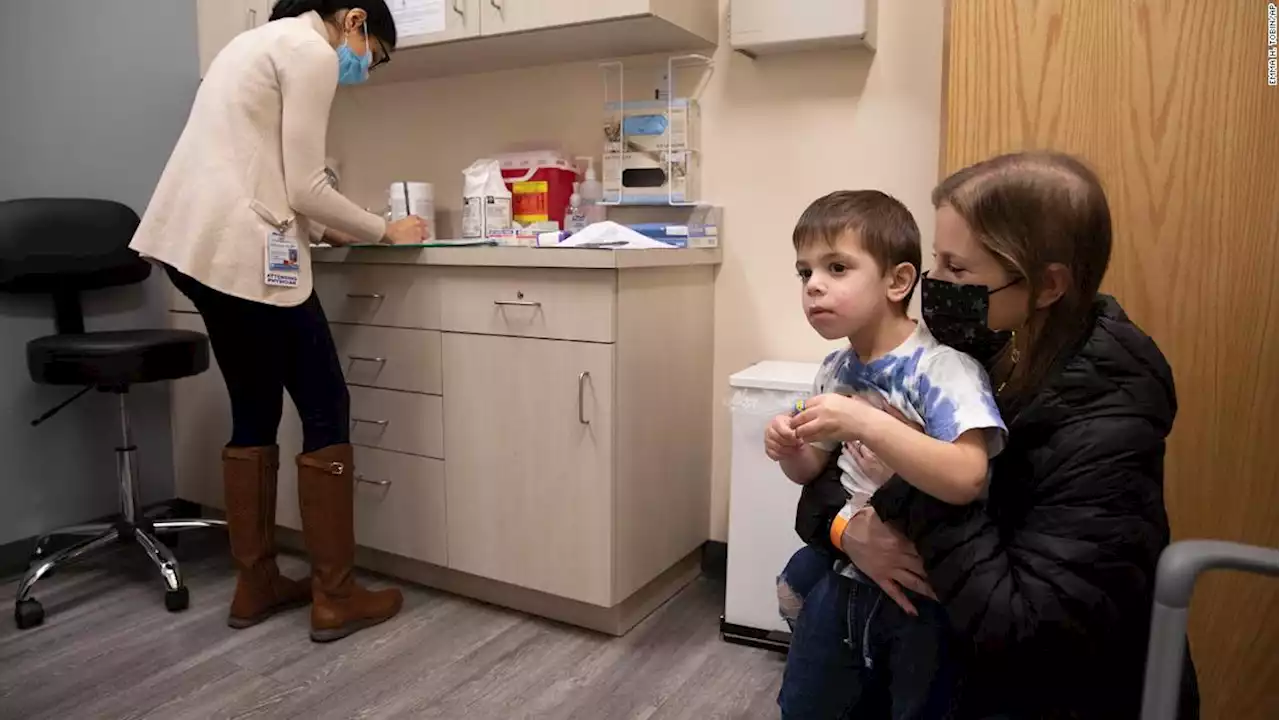 CDC advisers vote to recommend Covid-19 vaccines for children as young as 6 monthsVaccine advisers to the US Centers for Disease Control and Prevention voted unanimously on Saturday in support of recommending the Moderna and Pfizer/BioNTech Covid-19 vaccines to children as young as 6 months.
CDC advisers vote to recommend Covid-19 vaccines for children as young as 6 monthsVaccine advisers to the US Centers for Disease Control and Prevention voted unanimously on Saturday in support of recommending the Moderna and Pfizer/BioNTech Covid-19 vaccines to children as young as 6 months.
Consulte Mais informação »
