A green light from the CDC will pave the way for 3.2 million doses of the Novavax vaccine to be distributed in the United States.
A Centers for Disease Control and Prevention advisory panel voted Tuesday to recommend Novavax's two-dose Covid vaccine for adults.last week, but the CDC must sign off before doses can be distributed to the public. Now that the agency's advisory panel has weighed in affirmatively, it is up to CDC Director Dr. Rochelle Walensky to endorse the recommendation. In the past, that step has followed within the same day as the committee's vote.
Novavax’s vaccine stimulates the production of antibodies by introducing the body to a purified spike protein from the coronavirus, along with an immune-boosting ingredient called an adjuvant. The FDA has approved several protein-based vaccines in the past, including one for hepatitis B and another for shingles.
Novavax’s vaccine comes with another advantage, though: It can be stored in a regular refrigerator for up to six months, so it's easier to transport than mRNA vaccines, which must be kept at subzero temperatures.In a trial group of more than 30,000 people in the U.S. and Mexico, Novavax’s vaccine was found toby 90% and reduce the risk of moderate or severe disease by 100%.
Brasil Últimas Notícias, Brasil Manchetes
Similar News:Você também pode ler notícias semelhantes a esta que coletamos de outras fontes de notícias.
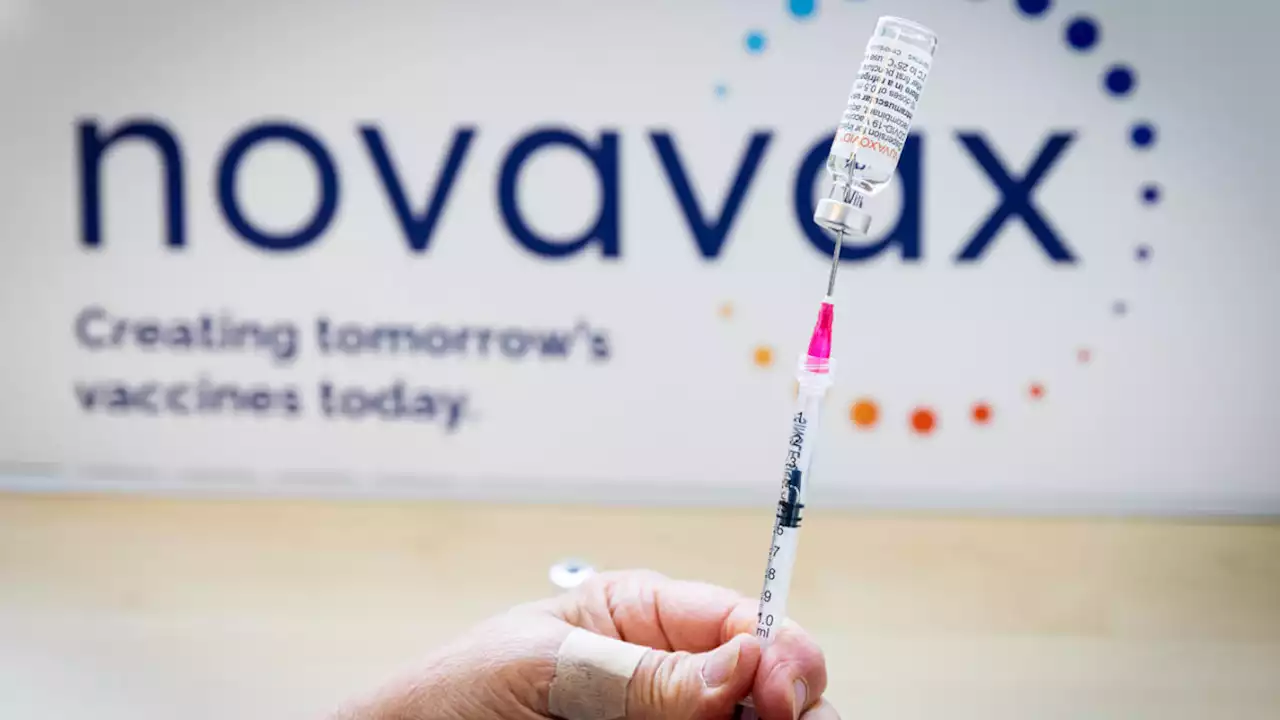 CDC panel endorses new Novavax COVID vaccineAdvisors to the Centers for Disease Control and Prevention voted Tuesday to endorse emergency authorization of a new COVID-19 vaccine developed by the Maryland biotechnology company Novavax, making it the fourth immunization to clear that hurdle in the U.S. — and the first to rely on the same familiar technology as some seasonal flu shots.
CDC panel endorses new Novavax COVID vaccineAdvisors to the Centers for Disease Control and Prevention voted Tuesday to endorse emergency authorization of a new COVID-19 vaccine developed by the Maryland biotechnology company Novavax, making it the fourth immunization to clear that hurdle in the U.S. — and the first to rely on the same familiar technology as some seasonal flu shots.
Consulte Mais informação »
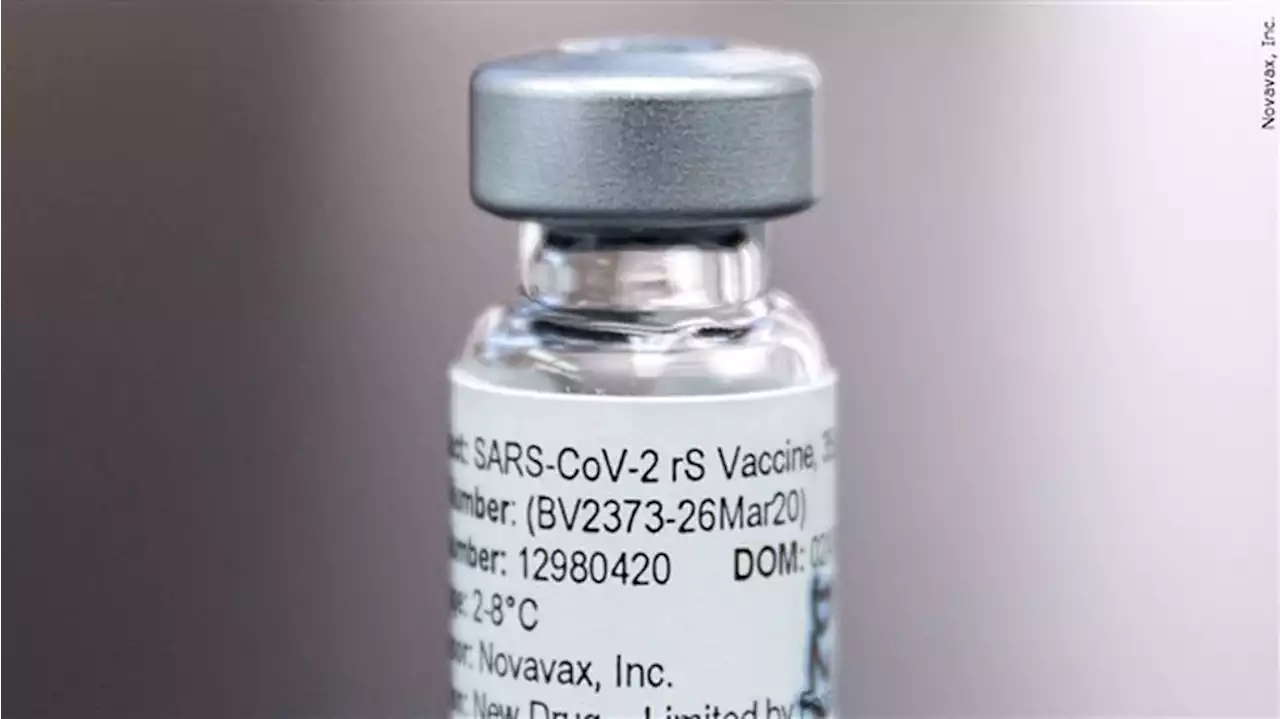 CDC advisers endorse more traditional Novavax COVID shotGovernment advisers say U.S. adults who haven’t gotten any COVID-19 shots yet should consider a new option from Novavax.
CDC advisers endorse more traditional Novavax COVID shotGovernment advisers say U.S. adults who haven’t gotten any COVID-19 shots yet should consider a new option from Novavax.
Consulte Mais informação »
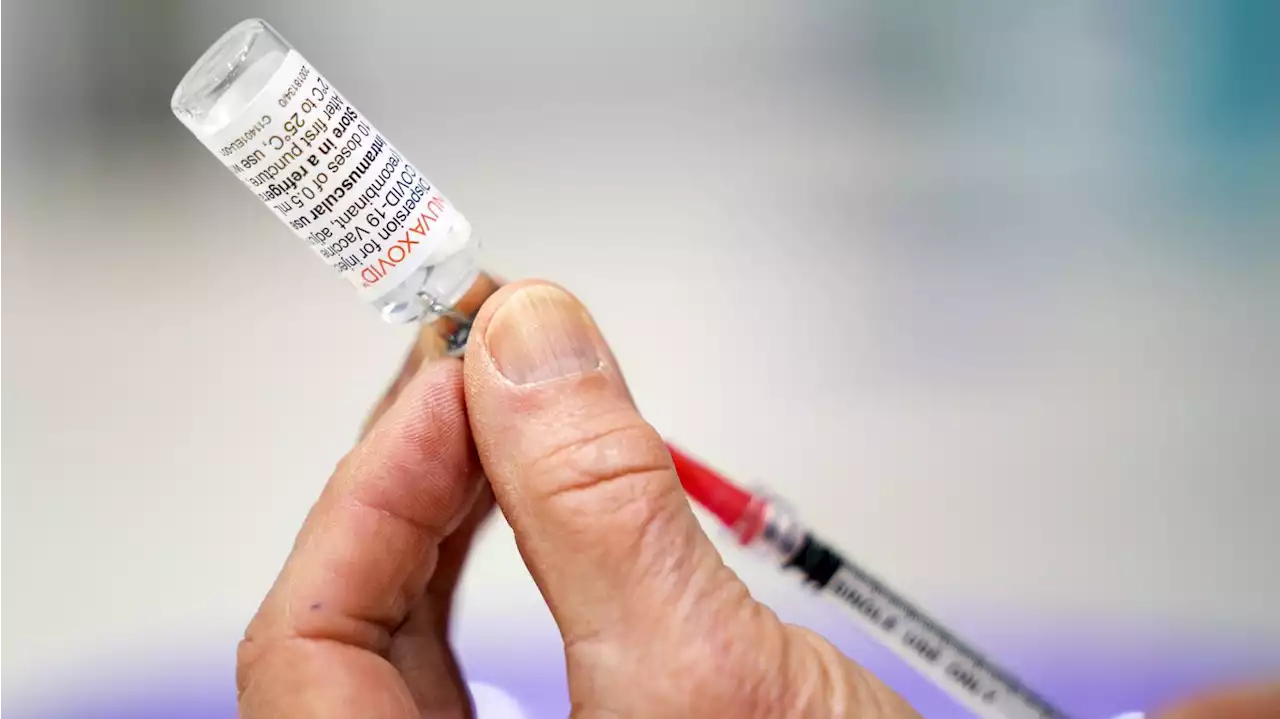 Novavax's COVID vaccine gets CDC panelIt is the fourth COVID shot authorized for use in the U.S.
Novavax's COVID vaccine gets CDC panelIt is the fourth COVID shot authorized for use in the U.S.
Consulte Mais informação »
 The CDC has ended its COVID-19 program for cruise shipsThe Centers for Disease Control and Prevention says that as of Monday, its COVID-19 program for cruise ships is no longer in effect, and the agency will no longer share each ship’s COVID status with the public.
The CDC has ended its COVID-19 program for cruise shipsThe Centers for Disease Control and Prevention says that as of Monday, its COVID-19 program for cruise ships is no longer in effect, and the agency will no longer share each ship’s COVID status with the public.
Consulte Mais informação »
 Experts Warn of Latest COVID-19 Subvariant as CDC Raises Dallas County Threat Level to RedDallas County is seeing a spike in COVID-19 cases thanks to the highly transmissible BA.5 subvariant.
Experts Warn of Latest COVID-19 Subvariant as CDC Raises Dallas County Threat Level to RedDallas County is seeing a spike in COVID-19 cases thanks to the highly transmissible BA.5 subvariant.
Consulte Mais informação »
 CDC ends COVID-19 monitoring program for cruise shipsCruise ships were among the first impacted by COVID-19 in 2020. The CDC issued no-sail orders, which kept ships at bay for months into the pandemic.
CDC ends COVID-19 monitoring program for cruise shipsCruise ships were among the first impacted by COVID-19 in 2020. The CDC issued no-sail orders, which kept ships at bay for months into the pandemic.
Consulte Mais informação »
